Abstract
Case Report
A Case of Catastrophic Atypical Hemolytic Uremic Syndrome Unresponsive to Eculizumab and the use of Ravulizumab Off-label
Jorge Cabrera Morales*, Giuseppe Sias, Marco Manzoni and Giacomina Loriga
Published: 05 October, 2023 | Volume 7 - Issue 3 | Pages: 073-077
“A 40-year-old woman with melanoma, under treatment with Dabrafenib and Trametinib, was evaluated in our hospital for rapidly progressive deterioration of renal function”.
8 months before the current admission, the patient had been diagnosed with melanoma, and underwent radical surgery and subsequent therapy with Dabrafenib and Trametinib.
After 5 months of therapy, the patient was brought to this hospital for precordial pain, with a diagnosis of myopericarditis, therapy was started for heart failure with a good response. However, the patient developed a progressive impairment of renal function, associated with hemolytic anemia and thrombocytopenia. The peripheral smear showed the presence of schistocytes.
The suspicion of atypical Hemolytic Uremic Syndrome (aHUS) was confirmed by the assay of C5B-9 induced by serum on endothelial cells, which showed a deposition of 331%, treatment with Eculizumab was initiated.
After 3 administrations the patient did not improve, with further worsening of the hemolytic condition, and progression of renal damage.
Due to the failure of Eculizumab, we considered the use of Ravulizumab. However, in Italy only can be administered to patients in Eculizumab stable treatment for at least three months. Nevertheless, faced with the catastrophic condition, it was decided to shift the therapy and use off-label Ravulizumab. After 10 days of the first administration, the laboratory tests showed a continuous rise in the values of haptoglobin, platelets, and hemoglobin, and a decrease in LDH. The renal function failed to return to normal values but after 20 days of therapy with Ravulizumab, there was complete resolution of the hemolytic condition.
Read Full Article HTML DOI: 10.29328/journal.jcn.1001113 Cite this Article Read Full Article PDF
Keywords:
Atypical hemolytic uremic syndrome; Myopericarditis; Eculizumab; Ravulizumab; Plasma exchange; C5 blockade; End-stage chronic kidney disease
References
- Mazzierli T, Allegretta F, Maffini E, Allinovi M. Drug-induced thrombotic microangiopathy: An updated review of causative drugs, pathophysiology, and management. Front Pharmacol. 2023 Jan 9;13:1088031. doi: 10.3389/fphar.2022.1088031. PMID: 36699080; PMCID: PMC9868185.
- Loirat C, Frémeaux-Bacchi V. Atypical hemolytic uremic syndrome. Orphanet J Rare Dis. 2011 Sep 8;6:60. doi: 10.1186/1750-1172-6-60. PMID: 21902819; PMCID: PMC3198674.
- Raina R, Krishnappa V, Blaha T, Kann T, Hein W, Burke L, Bagga A. Atypical Hemolytic-Uremic Syndrome: An Update on Pathophysiology, Diagnosis, and Treatment. Ther Apher Dial. 2019 Feb;23(1):4-21. doi: 10.1111/1744-9987.12763. Epub 2018 Oct 29. PMID: 30294946.
- Tschumi S, Gugger M, Bucher BS, Riedl M, Simonetti GD. Eculizumab in atypical hemolytic uremic syndrome: long-term clinical course and histological findings. Pediatr Nephrol. 2011 Nov;26(11):2085-8. doi: 10.1007/s00467-011-1989-4. Epub 2011 Aug 30. PMID: 21877169.
- Nishimura J, Yamamoto M, Hayashi S, Ohyashiki K, Ando K, Brodsky AL, Noji H, Kitamura K, Eto T, Takahashi T, Masuko M, Matsumoto T, Wano Y, Shichishima T, Shibayama H, Hase M, Li L, Johnson K, Lazarowski A, Tamburini P, Inazawa J, Kinoshita T, Kanakura Y. Genetic variants in C5 and poor response to eculizumab. N Engl J Med. 2014 Feb 13;370(7):632-9. doi: 10.1056/NEJMoa1311084. PMID: 24521109.
- McKeage K. Ravulizumab: First Global Approval. Drugs. 2019 Feb;79(3):347-352. doi: 10.1007/s40265-019-01068-2. PMID: 30767127.
- Connelly-Smith L, Alquist CR, Aqui NA, Hofmann JC, Klingel R, Onwuemene OA, Patriquin CJ, Pham HP, Sanchez AP, Schneiderman J, Witt V, Zantek ND, Dunbar NM. Guidelines on the Use of Therapeutic Apheresis in Clinical Practice - Evidence-Based Approach from the Writing Committee of the American Society for Apheresis: The Ninth Special Issue. J Clin Apher. 2023 Apr;38(2):77-278. doi: 10.1002/jca.22043. PMID: 37017433.
- Lee JW, Sicre de Fontbrune F, Wong Lee Lee L, Pessoa V, Gualandro S, Füreder W, Ptushkin V, Rottinghaus ST, Volles L, Shafner L, Aguzzi R, Pradhan R, Schrezenmeier H, Hill A. Ravulizumab (ALXN1210) vs eculizumab in adult patients with PNH naive to complement inhibitors: the 301 study. Blood. 2019 Feb 7;133(6):530-539. doi: 10.1182/blood-2018-09-876136. Epub 2018 Dec 3. PMID: 30510080; PMCID: PMC6367644.
- Banks M, Crowell K, Proctor A, Jensen BC. Cardiovascular Effects of the MEK Inhibitor, Trametinib: A Case Report, Literature Review, and Consideration of Mechanism. Cardiovasc Toxicol. 2017 Oct;17(4):487-493. doi: 10.1007/s12012-017-9425-z. PMID: 28861837; PMCID: PMC6319910.
- Banks M, Crowell K, Proctor A, Jensen BC. Cardiovascular Effects of the MEK Inhibitor, Trametinib: A Case Report, Literature Review, and Consideration of Mechanism. Cardiovasc Toxicol. 2017 Oct;17(4):487-493. doi: 10.1007/s12012-017-9425-z. PMID: 28861837; PMCID: PMC6319910.
- Lyon AR, López-Fernández T, Couch LS, Asteggiano R, Aznar MC, Bergler-Klein J, Boriani G, Cardinale D, Cordoba R, Cosyns B, et al. ESC Scientific Document Group. 2022 ESC Guidelines on cardio-oncology developed in collaboration with the European Hematology Association (EHA), the European Society for Therapeutic Radiology and Oncology (ESTRO) and the International Cardio-Oncology Society (IC-OS). Eur Heart J. 2022 Nov 1;43(41):4229-4361. doi: 10.1093/eurheartj/ehac244. Erratum in: Eur Heart J. 2023 May 7;44(18):1621. PMID: 36017568.
- Reese JA, Bougie DW, Curtis BR, Terrell DR, Vesely SK, Aster RH, George JN. Drug-induced thrombotic microangiopathy: Experience of the Oklahoma Registry and the BloodCenter of Wisconsin. Am J Hematol. 2015 May;90(5):406-10. doi: 10.1002/ajh.23960. Epub 2015 Feb 25. PMID: 25639727; PMCID: PMC4409501.
- Akino S, Ohashi H, Okano T, Takeuchi S, Kawakami T, Soma Y, Kadono T. Sudden elevation of plasma D-dimer levels induced by the combination therapy of dabrafenib and trametinib: Report of two cases. J Dermatol. 2019 Apr;46(4):358-360. doi: 10.1111/1346-8138.14798. Epub 2019 Feb 5. PMID: 30719722.
- Zafar A, Lim MY, Abou-Ismail MY. Eculizumab in the management of drug-induced thrombotic microangiopathy: A scoping review of the literature. Thromb Res. 2023 Apr;224:73-79. doi: 10.1016/j.thromres.2023.02.012. Epub 2023 Mar 2. PMID: 36871347.
- Rondeau E, Scully M, Ariceta G, Barbour T, Cataland S, Heyne N, Miyakawa Y, Ortiz S, Swenson E, Vallee M, Yoon SS, Kavanagh D, Haller H; 311 Study Group. The long-acting C5 inhibitor, Ravulizumab, is effective and safe in adult patients with atypical hemolytic uremic syndrome naïve to complement inhibitor treatment. Kidney Int. 2020 Jun;97(6):1287-1296. doi: 10.1016/j.kint.2020.01.035. Epub 2020 Mar 6. Erratum in: Kidney Int. 2020 Dec;98(6):1621. Erratum in: Kidney Int. 2021 May;99(5):1244. PMID: 32299680.
- Peffault de Latour R, Brodsky RA, Ortiz S, Risitano AM, Jang JH, Hillmen P, Kulagin AD, Kulasekararaj AG, Rottinghaus ST, Aguzzi R, Gao X, Wells RA, Szer J. Pharmacokinetic and pharmacodynamic effects of ravulizumab and eculizumab on complement component 5 in adults with paroxysmal nocturnal haemoglobinuria: results of two phase 3 randomised, multicentre studies. Br J Haematol. 2020 Nov;191(3):476-485. doi: 10.1111/bjh.16711. Epub 2020 May 24. PMID: 32449174; PMCID: PMC7687070.
- Aringer M, Costenbader K, Daikh D, Brinks R, Mosca M, Ramsey-Goldman R, Smolen JS, Wofsy D, Boumpas DT, Kamen DL, Jayne D, Cervera R, Costedoat-Chalumeau N, et al. 2019 European League Against Rheumatism/American College of Rheumatology Classification Criteria for Systemic Lupus Erythematosus. Arthritis Rheumatol. 2019 Sep;71(9):1400-1412. doi: 10.1002/art.40930. Epub 2019 Aug 6. PMID: 31385462; PMCID: PMC6827566.
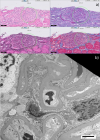
Figures:

Figure 1
Similar Articles
-
Posterior Reversible Leukoencephalopathy Syndrome in a patient after second dose of Rituximab for treatment of resistant Thrombotic Thrombocytopenic PurpuraSabaa Asif*,Sumbal Nasir Mahmood,Osama Kunwer Naveed. Posterior Reversible Leukoencephalopathy Syndrome in a patient after second dose of Rituximab for treatment of resistant Thrombotic Thrombocytopenic Purpura . . 2018 doi: 10.29328/journal.jcn.1001010; 2: 001-004
-
Complete recovery of chronic Osmotic Demyelination Syndrome with plasma exchangeSumbal Nasir Mahmood*,Kunwer Naveed Mukhtar,Osama Kunwer Naveed,Ahmed Kunwer Naveed. Complete recovery of chronic Osmotic Demyelination Syndrome with plasma exchange. . 2018 doi: 10.29328/journal.jcn.1001011; 2: 005-007
-
SGLT2 Inhibitors and nephroprotection in diabetic kidney disease: From mechanisms of action to the latest evidence in the literatureJorge Rico-Fontalvo,Rodrigo Daza-Arnedo,Maria Ximena Cardona-Blanco,Victor Leal-Martínez,Emilio Abuabara-Franco,Nehomar Pajaro-Galvis*,Jose Cabrales,José Correa,Manuel Cueto,Amable Duran,Alejandro Castellanos,Javier Enamorado,José Bohórquez,Isabella Uparella,Julio Zuñiga,Abraham Chagui,Alfonso Ramos,Luis Lara. SGLT2 Inhibitors and nephroprotection in diabetic kidney disease: From mechanisms of action to the latest evidence in the literature. . 2020 doi: 10.29328/journal.jcn.1001058; 4: 044-055
-
Prostate cancer-associated thrombotic microangiopathy: A case report and review of the literatureHHS Kharagjitsing*,PAW te Boekhorst,Nazik Durdu-Rayman. Prostate cancer-associated thrombotic microangiopathy: A case report and review of the literature. . 2021 doi: 10.29328/journal.jcn.1001068; 5: 017-022
-
Impact of a multidisciplinary pre-dialysis program on renal treatment modalities choiceLuis Miguel Castro Fonseca dos Santos Oliveira*,Rui Arlindo dos Santos Alves de Castro,Teresa Margarida Ribeiro Pinto Morgado. Impact of a multidisciplinary pre-dialysis program on renal treatment modalities choice. . 2021 doi: 10.29328/journal.jcn.1001073; 5: 047-052
-
Convalescent plasma therapy in aHUS patient with SARS-CoV-2 infectionEmma Diletta Stea*,Virginia Pronzo,Francesco Pesce,Marco Fiorentino,Adele Mitrotti,Vincenzo Di Leo,Cosma Cortese,Annalisa Casanova,Sebastiano Nestola,Flavia Capaccio,Loreto Gesualdo. Convalescent plasma therapy in aHUS patient with SARS-CoV-2 infection. . 2022 doi: 10.29328/journal.jcn.1001088; 6: 036-039
-
A Case of Catastrophic Atypical Hemolytic Uremic Syndrome Unresponsive to Eculizumab and the use of Ravulizumab Off-labelJorge Cabrera Morales*, Giuseppe Sias, Marco Manzoni, Giacomina Loriga. A Case of Catastrophic Atypical Hemolytic Uremic Syndrome Unresponsive to Eculizumab and the use of Ravulizumab Off-label. . 2023 doi: 10.29328/journal.jcn.1001113; 7: 073-077
-
Atypical Anti-GBM with ANCA Vasculitis- A Rarest of the Rare Entity: Index Case from Eastern IndiaGopambuj Singh Rathod*, Atanu Pal, Pallavi Mahato, Aakash Roy, Debroop Sengupta, Muzzamil Ahmad. Atypical Anti-GBM with ANCA Vasculitis- A Rarest of the Rare Entity: Index Case from Eastern India. . 2024 doi: 10.29328/journal.jcn.1001139; 8: 124-126
-
Double-Positive Anti-GBM and ANCA Vasculitis: 2 Case Reports and Review of the LiteratureBenhadda Selim*,Nmili Manal,Nassiri Nada,Benamar Loubna,Ouzeddoun Naima,Bouattar Tarik. Double-Positive Anti-GBM and ANCA Vasculitis: 2 Case Reports and Review of the Literature. . 2025 doi: 10.29328/journal.jcn.1001145; 9: 009-012
Recently Viewed
-
Environmental Factors Affecting the Concentration of DNA in Blood and Saliva Stains: A ReviewDivya Khorwal*, GK Mathur, Umema Ahmed, SS Daga. Environmental Factors Affecting the Concentration of DNA in Blood and Saliva Stains: A Review. J Forensic Sci Res. 2024: doi: 10.29328/journal.jfsr.1001057; 8: 009-015
-
Markov Chains of Molecular Processes of Biochemical MaterialsOrchidea Maria Lecian*. Markov Chains of Molecular Processes of Biochemical Materials. Int J Phys Res Appl. 2024: doi: 10.29328/journal.ijpra.1001076; 7: 001-005
-
Generation of Curved Spacetime in Quantum FieldSarfraj Khan*. Generation of Curved Spacetime in Quantum Field. Int J Phys Res Appl. 2024: doi: 10.29328/journal.ijpra.1001077; 7: 006-009
-
Optimizing Milk Safety: Applying Nuclear Techniques in X-ray Fluorescence Spectroscopy for Heavy Metal Quantification in Powdered Milk Consumed in SenegalPapa Macoumba Faye*, Djicknack Dione, Oumar Ndiaye, Moussa Hamady SY, Nogaye Ndiaye, Alassane Traore, Ababacar Sadikhe Ndao. Optimizing Milk Safety: Applying Nuclear Techniques in X-ray Fluorescence Spectroscopy for Heavy Metal Quantification in Powdered Milk Consumed in Senegal. Int J Phys Res Appl. 2024: doi: 10.29328/journal.ijpra.1001078; 7: 010-015
-
Thermoelectric Materials Based on Lead Telluride and Prospects for their Practical ApplicationYuriy Pavlovskyy*, Nadiya Pavlovska. Thermoelectric Materials Based on Lead Telluride and Prospects for their Practical Application. Int J Phys Res Appl. 2024: doi: 10.29328/journal.ijpra.1001079; 7: 016-018
Most Viewed
-
Evaluation of Biostimulants Based on Recovered Protein Hydrolysates from Animal By-products as Plant Growth EnhancersH Pérez-Aguilar*, M Lacruz-Asaro, F Arán-Ais. Evaluation of Biostimulants Based on Recovered Protein Hydrolysates from Animal By-products as Plant Growth Enhancers. J Plant Sci Phytopathol. 2023 doi: 10.29328/journal.jpsp.1001104; 7: 042-047
-
Sinonasal Myxoma Extending into the Orbit in a 4-Year Old: A Case PresentationJulian A Purrinos*, Ramzi Younis. Sinonasal Myxoma Extending into the Orbit in a 4-Year Old: A Case Presentation. Arch Case Rep. 2024 doi: 10.29328/journal.acr.1001099; 8: 075-077
-
Feasibility study of magnetic sensing for detecting single-neuron action potentialsDenis Tonini,Kai Wu,Renata Saha,Jian-Ping Wang*. Feasibility study of magnetic sensing for detecting single-neuron action potentials. Ann Biomed Sci Eng. 2022 doi: 10.29328/journal.abse.1001018; 6: 019-029
-
Pediatric Dysgerminoma: Unveiling a Rare Ovarian TumorFaten Limaiem*, Khalil Saffar, Ahmed Halouani. Pediatric Dysgerminoma: Unveiling a Rare Ovarian Tumor. Arch Case Rep. 2024 doi: 10.29328/journal.acr.1001087; 8: 010-013
-
Physical activity can change the physiological and psychological circumstances during COVID-19 pandemic: A narrative reviewKhashayar Maroufi*. Physical activity can change the physiological and psychological circumstances during COVID-19 pandemic: A narrative review. J Sports Med Ther. 2021 doi: 10.29328/journal.jsmt.1001051; 6: 001-007

HSPI: We're glad you're here. Please click "create a new Query" if you are a new visitor to our website and need further information from us.
If you are already a member of our network and need to keep track of any developments regarding a question you have already submitted, click "take me to my Query."