Research Article
Anemia response to Methoxy Polyethylene Glycol-Epoetin Beta (Mircera) versus Epoetin Alfa (Eprex) in patients with chronic Kidney disease on Hemodialysis

Alaa K Dhayef*, Jawad K Manuti and Abdulwahab S Abutabiekh
Department Of Medicine, F.I.C.M.S AL-Nahrain University-Medical College Institution, Baghdad, Iraq
*Address for Correspondence: Dr. Jawad K Manuti, Professor, F.I.C.M.S AL-Nahrain University-Medical College Institution, Department of Medicine, Dialysis Unit, IRAQ-Baghdad, Iraq, Tel: 9647801642234; Email: drjawadkadhem@yahoo.com
Dates: Submitted: 24 June 2017; Approved: 31 August 2017; Published: 05 September 2017
How to cite this article: Dhayef AK, Manuti JK, Abutabiekh AS. Anemia response to Methoxy Polyethylene Glycol-Epoetin Beta (Mircera) versus Epoetin Alfa (Eprex) in patients with chronic Kidney disease on Hemodialysis. J Clini Nephrol. 2017; 1: 041-047. DOI: 10.29328/journal.jcn.1001006
Copyright License: © 2017 Dhayef AK, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: Mircera; Eprex; Hemodialysis
Abstract
Objective: Anemia, a common complication of chronic kidney disease, usually develops because of erythropoietin deficiency. Maintaining target hemoglobin (Hb) with minimal variability is a challenge in hemodialysis (HD) patients. The aim of this study is to compare the long- and short-acting erythropoietin erythropoietin stimulating agents such as Mircera and Eprex in achieving these targets.
Results: The response rate in the evaluation period was higher in patients treated with methoxypolyethylene glycol-epoetin beta (Mircera) than with epoetin (Eprex) alfa: 36 of 50 (72%) mean Hb concentration (10.51g/dl) versus 29 of 50 (58%) mean Hb concentration (9.81), with statistically significant p-value <0.0001.
Conclusion: Treatment with (Mircera) administered intravenously once monthly was superior to treatment with (Eprex) administered subcutaneously three times weekly for maintaining haemoglobin concentrations in patients with chronic kidney disease on hemodialysis.
Introduction
Anaemia is a condition in which the number of red blood cells or their oxygen-carrying capacity is insufficient to meet physiologic needs, which vary by age, sex, altitude, smoking, and pregnancy status. According to the World Health Organization (WHO), anemia is defined as a hemoglobin level of less than 13g/dl in men and less than 12 g/dl in women [1-4]. Several mechanisms are implicated in anemia in chronic renal failure including deficiency of erythropoietin, toxic effects of uraemia on marrow precursor cells, reduced red cell survival, increased blood loss due to capillary fragility and poor platelet function and reduced intake, absorption and utilization of dietary iron [5]. The anemia of CKD is associated with number of adverse pathophysiologic consequences, including decreased tissue oxygen delivery and utilization, increased cardiac output, ventricular dilation, and ventricular hypertrophy. Clinical manifestation includes fatigue and diminished exercise tolerance, angina, heart failure, decreased cognition and mental acuity and impaired host defense against infection [6]. Anaemia is common in patients with a GFR below 30 ml/min/1.73m2 and contributes to many of the specific symptoms of CKD. Recombinant human erythropoietin is effective in correcting the anaemia of CKD and improving the associated morbidity.
Erythropoietin treatment does not influence mortality, however correcting haemoglobin to normal levels may carry some extra risk, including hypertension and thrombosis (including thrombosis of the arteriovenous.
Fistulae used for haemodialysis). Erythropoietin is less effective in the presence of iron deficiency, active inflammation or malignancy, and in patients with aluminum overload, which may occur in dialysis [5-7].
However, evidence suggesting that the correction of anemia improves cardiovascular outcomes has largely been derived from observational studies and small interventional trials associating a high level of. Hemoglobin (>12.0 g per deciliter) with a lower rate of complications and death from cardiovascular causes [8-10]. Other evidence has also indicated that cardiovascular complications, such as left ventricular hypertrophy, might be improved through the use of a high hemoglobin. Level as a target. However, in a randomized, controlled study comparing a hematocrit target of 42% with that of 30% among patients with heart disease who were undergoing hemodialysis, the former group had higher. Rates of nonfatal myocardial infarction and death, but not significantly so [11]. Approved erythropoiesis-stimulating agents with short half-lives generally require short dosing intervals and frequent administration (mainly two to three times per week) to maintain stable haemoglobin concentrations [12]. An erythropoiesis-stimulating agent with a longer half-life and correspondingly longer dose interval might improve anaemia management and provide greater convenience to patients while.
Decreasing the impact on health-care resources [13]. This has led to clinical testing of available erythropoiesis stimulating agents at dosing intervals longer than those in approved labelling. However, most published studies. Tested dosing intervals shorter than once monthly and used uncontrolled designs [14,15], two types of recombinant human erythropoietin. (Epoetin alfa and epoetin beta) have been available since ESAs first came into use; both types are highly effective but short-acting (approved for dosing three times a week). Subsequently, two second-generation ESAs.
With an extended duration of action were developed: Darbepoetin alfa, which has an altered glycosylation pattern, and a continuous erythropoietin receptor activator called methoxy polyethylene glycol-epoetin beta (PEG-EPO) (Mircera, Hoffmann-La Roche), which contains a polyethylene-glycol moiety. Darbepoetin alfa is approved for dosing every 2 weeks worldwide, and PEG-EPO for dosing once a month in Europe [16]. The haemoglobin concentration should be maintained within the range 10-12g/100ml; haemoglobin concentrations higher than 12g/100ml should be avoided (in some patients, this may be achieved at concentrations lower than the recommended range) [17-19].
Methods
The study was performed in Al-Nahrain College of Medicine in Al-Kadhmiya Teaching Hospital in dialysis unit during the period of May 2015 to December 2015. One hundred patients (56 males and 44 females) involved in this study of different age groups ranging from 15to70 years (mean of age 47.6 years) complaining of chronic renal failure on regular hemodialysis, each patient subjected to hemodialysis for period of 4 hours in two or three sessions per week using GAMBRO AK95S haemodialysis apparatus with polyfluxTML dialyzer membrane with effective surface area range from 1.4 to 2.1 m2 and flow rate range from 250 to 300 ml/min. Patients on hemodialysis were required to have dialysis adequacy (Kt/v) of 1.2 or more. All patients involved in the study had hemoglobin range from 7-12 g/dl and divided into two groups, the first group have undergone continuous 2-3 times weekly maintenance subcutaneous epoetin alfa (Eprex is the tradename in Australia and New Zealand for Epoetin alfa (rch) (r-HuEPO) (JANSSEN-CILAG Pty Ltd) therapy for 6 months, the second group received intravenous methoxy polyethylene glycol <8000 IU of epoetin alfa per week, the starting dose of methoxy polyethylene glycol-epoetin beta was 120μg. Patients who had received 8000-16000 IU or >16000 IU of epoetin alfa per week were given 200 or 360μg of methoxy polyethylene glycol-epoetin beta, respectively. Doses of methoxy polyethylene glycol-epoetin beta were to be decreased by 25% for haemoglobin >13 and ≤14g/dl and increased by 25% for haemoglobin decreases >1g/dl versus baseline or for haemoglobin <11 and ≥9g/dl. Methoxy polyethylene glycol-epoetin beta dose decreases of 50% were to be made for haemoglobin increases >2g/dl versus baseline and increases of 50% for haemoglobin decreases >2g/dl versus baseline or hemoglobin <9g/d. Epoetin alfa doses were to be adjusted according to the approved prescribing information, without additional restrictions. Iron supplementation was to be initiated or intensified according to center practice in cases of iron deficiency (serum ferritin <100μg/l, transferrin saturation <20%, or hypochromic red blood cells >10%) and discontinued in patients who had serum ferritin levels >800μg/l or transferrin saturation >50%. All patients underwent a history and physical examination and asked whether they had experienced any adverse events. Blood pressure, heart rate, complete blood picture include (hemoglobin and hematocrit, white blood cell count and differential and platelets) were measured at every visit. Aspartate amino transferees, alanine aminotransferase, albumin, alkaline phosphatase, potassium, phosphorus, serum ferritin, serum iron, serum transferrin, transferrin saturation, total iron binding capacity or proportion of hypochromic red blood cells were measured every month.
Those who missed three hemodialysis sessions or more, bleeding complication, infectious disease, had nonrenal causes of anemia (e.g. hemoglobinopathies, hemolysis, or vitamin B12 or folic acid deficiency), any-epoetin beta (Mircera) (F. Hoffmann-La Roche, Basel, Switzerland) once monthly. Patients also had to have adequate iron status, defined as serum ferritin ≥100 g/l, transferrin saturation ≥20%. For patients who previously received patient with hyperkalemia, abnormal white cell count, platelets, and all patients required blood transfusion were excluded from the study. The FDA has determined that the target hemoglobin (Hb) level for patients with anemia receiving erythropoiesis-stimulating agents (ESAs) is 10 to 12g/dl.19.
Statistical Analysis
Results are expressed as mean±standard deviation (SD) values. We assessed differences in the same group using the t-test for paired data. The unpaired t-test was used to compare mean values between groups. Repeated ANOVA was used to compare median changes between groups. SPSS Statistics ver.23 (IBM Japan, Tokyo, Japan) was used for statistical analysis. Significance was established at a level of p<0.05.
Results
All patients involved in this study had hemoglobin level range from 7 to 12, most of them were take methoxy polyethylene glycol-epoetin beta or epoetin alfa for correction of anemia which results from chronic kidney disease (although the patients had adequate dialysis Kt/v more than 1.2).
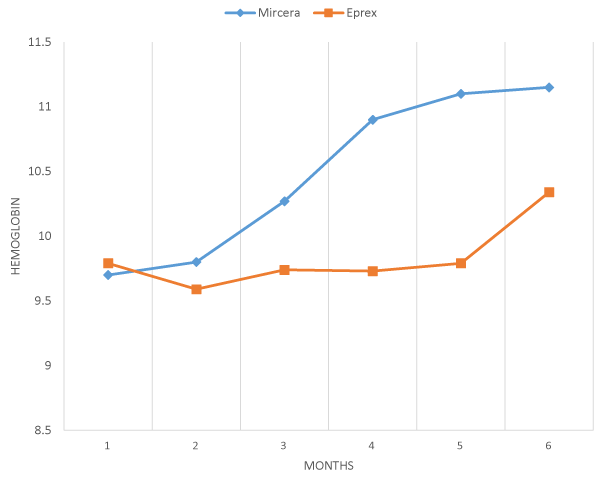
The data of 100 hemodialysis patients were analyzed, 50 (50%) were in Mircera group and 50 (50%) were in Eprex group. The mean age was 45.66 (17-70 years), and there were 56 (56%) males. Diabetes mellitus was the major cause of ESRD and encountered in 30 (30%) of the patients. The majority of patients 86 (86%) had no viral hepatitis while 14 patients (14%) were HCV positive. The comparison between Mircera and Eprex in each month during 6 months duration time of follow up in this study shows no statistically significant difference, but shows that there is good response of hemoglobin in first patients group that received Mircera while less response in second group which received Eprex in the 6months of follow up, which is clear in the last 3 months, as mentioned in table 1 and figure 1.
| Table 1: Baseline demographic and characteristic of the patients | |||
| Age(years) | 17-70 | 45.66±12.27 | |
| Weight (kg) | 23-85 | 56.39±15.15 | |
| Sex | Male | 56(56%) | |
| Female | 44(44%) | ||
| HCV | Positive | 14(14%) | |
| Negative | 86(86%) | ||
| Hemoglobin | 7-12 g/dl | 10.27±2.36 | |
| Kt/v | 1.2-1.4 | 1.35±0.21 | |
| Albumin | 3.1-4.48 g/dl | 3.65±0.30 | |
| Parathyroid Hormone | 34.9-1188 Pg./ml | 364.3±264.18 | |
| Creatinine | 3.28-11.38 mg/dl | 7.86±2.2 | |
| Ferritin | 148.7-2000 ng/ml | 846.3±569.5 | |
| Transferrin Saturation | 11-87 % | 33.92±14.21 | |
| Eprex | 50 | ||
| Mircera | 50 | ||
| Cause of CKD | DM | 35(30%) | |
| HT | 2 6(28%) | ||
| GN | 6(6%) | ||
| PCK | 7 (7%) | ||
| Obstructive | 1 0(12%) | ||
| vasculitis | 6(%) | ||
| pyelonephritis | 7(6%) | ||
Figure 1: Mean hemoglobin value in correction period in response to mircera once monthly and eprex three times weekly.
According to the cause of chronic kidney disease (CKD) the most common cause of CKD is diabetic patients and 2nd common cause is hypertension but unknown causes represent 3% of all causes in this study as mention in table 2,3.
| Table 2: Mean hemoglobin concentration according to causes of CKD in each group. | |||
| CAUSES OF CKD | Drug | No. of patients | Hemoglobin mean±SD |
| Diabetes mellitus | eprex | 15 | 10.35±0.55 |
| mircera | 20 | 10.65±1.03 | |
| Hypertension | Eprex | 15 | 9.80±1.03 |
| Mircera | 11 | 9.66±0.60 | |
| glomerulonephritis | Eprex | 4 | 8.78±0.12 |
| Mircera | 2 | 10.17±0.0 | |
| Poly cystic kidney | Eprex | 3 | 9.33±1.17 |
| Mircera | 4 | 10.05±0.55 | |
| obstruction | Eprex | 3 | 9.40±1.13 |
| Mircera | 7 | 10.70±0.96 | |
| vasculitis | eprex | 4 | 9.6±1.01 |
| Mircera | 2 | 10.1±0.52 | |
| pyelonephritis | Eprex | 4 | 10.3±0.57 |
| Mircera | 3 | 9.41±0.34 | |
| unknown | Eprex | 1 | 9.80±1.47 |
| mircera | 2 | 10.86±0.70 | |
| Total | 100 | ||
| Table 3: Comparison of mean hemoglobin concentration in mircera group and eprex group each month during the study follow up period 6 months. | |||||
| Variable | Month | Drugs | No. of patients | Mean ±SD | p value |
| Hemoglobin | 1st | Mircera | 50 | 9.8±1.43 | 0.66 |
| eprex | 50 | 9.59±1.65 | |||
| 2nd | Mircera | 50 | 9.7±1.45 | 0.86 | |
| Eprex | 50 | 9.79±1.22 | |||
| 3rd | Mircera | 50 | 10.27±1.11 | 0.41 | |
| Eprex | 50 | 9.74±1.26 | |||
| 4th | Mircera | 50 | 10.9±1.26 | 0.892 | |
| Eprex | 50 | 9.73±1.21 | |||
| 5th | Mircera | 50 | 11.1±1.12 | 0.316 | |
| Eprex | 50 | 9.79±1.23 | |||
| 6th | Mircera | 50 | 11.15±0.72 | 0.140 | |
| Eprex | 50 | 10.34±0.93 | |||
The response rate in the evaluation period was higher in patients treated with methoxy polyethylene glycolepoetin beta (Mircera) than with epoetin (Eprex) alfa: 36 out of 50 (72%) mean Hb concertation (10.51g/dl) versus 29 of 50 (58%) mean Hb concentration (9.81g/dl), with statistically significant p<0.0001. Other biological tests did not significantly differ in both groups, there was no effect of serum ferritin, transferrin saturation, serum albumin, adequacy of dialysis, serum creatinine and parathyroid hormone in response to the Mircera versus Eprex in the treatment of anemia in patients treated by hemodialysis as mention in table 4.
| Table 4: Biological parameter in each group. | |||
| Laboratory characters | MIRCERA | EPREX | P VALUE |
| MEAN±SD | MEAN±SD | ||
| HEMOGLOBIN (g/dl) | 10. 51±1.44 | 9.81±1.45 | ≤0.0001 |
| TRANSFERRIN SATURATION (%) | 33.39±16.96 | 33.56±10.8 | 0.68 |
| FERRITINE (ng/ml) | 725±428 | 780±513 | 0.168 |
| Kt/v | 1.34±0.24 | 1.34±0.18 | 0.15 |
| S. CREATININE (mg/dl) | 7.5±2.36 | 8.05±2.03 | 0.329 |
| S. ALBUMIN (g/dl) | 3.67±0.26 | 3.58±0.4 | 0.164 |
| PARATHYROID HORMON (pg./ml) | 362.5±284 | 354.7±225 | 0.729 |
Regarding to sex variation according to correction of anemia and reach of hemoglobin to average (10-12.5g/dl) was no significant in both group (p value 0.239). number of patients that anemia corrected in mircera group were male 18of 27 and female 16 of 23, while in eprex group male 18 of 29 and female 12 of 21, which is statistically no significant.as mention in table 5.
| Table 5: Sex variation and Correction of anemia in each group. | ||||||
| Drug | Hemoglobin | Total | P value | |||
| Anemia NO (%) |
Corrected NO (%) |
|||||
| Mircera | SEX | Male | 9(33.3%) | 18(66.7%) | 27 | 0.208 |
| Female | 7(30.3%) | 16(69.7%) | 23 | |||
| Total | 16(28%) | 34(68%) | 50 | |||
| Eprex | SEX | Male Female |
11(37.9%) 9(42.9%) |
18(62.1%) 12(57.1%) |
29 21 |
0.222 |
| Total | 20(40%) | 30 (60%) | 50 | |||
| Total | SEX | Male | 20(32.2%) | 36(67.8%) | 56 | 0.239 |
| Female | 16(36.4%) | 28(63.6%) | 44 | |||
| Total | 36(34%) | 64(64%) | 100 | |||
Discussion
We studied the efficacy of methoxy polyethylene glycol-epoetin beta in treatment of anemic patient in chronic kidney disease on hemodialysis as compared with other ESAs (Eprex). The target of hemoglobin in patients with CKD on hemodialysis was 10-11.5 g/dl, 6 but evidence suggests that only 63% of patients reach the target range. The proportion of all patients who entered the study and met the response criteria of no hemoglobin decrease from baseline exceeding 1 g/dl and average hemoglobin ≥10.5 g/dl during the final evaluation period almost 6 months later was significantly higher in patients treated with Mircera than in those with given Eprex (mean hemoglobin concertation (10.51) in Mircera group and (9.81) in the Eprex group, p-value ≤0.001 as mentioned in Table 3.The results of this study show that both ESAs maintained the target hemoglobin in almost 64% of the patients compared with 72.83% in (a randomized comparative trial)20 and 40% in large dialysis US patients.21 However, the results illustrate the impact of type of ESAs used in maintaining target hemoglobin, there was significant difference between the percentage of patients achieving target hemoglobin in Mircera group (72%) compared to those in Eprex group (58%), Table 4. Hemoglobin variability assessed by different statistical methods showed a tendency of better Hb stability in the Mircera group compared to the Eprex group with statistically significant difference between the two groups (p-value <0.0001), Table 3.All biological parameters (ferritin, transferrin saturation, serum albumin, parathyroid hormone, efficacy of dialysis (Kt/v) and serum creatinine) evaluated during the study were comparable between the two groups, and there was no difference between the baseline and end of the study parameters.
Sex variation in this study shows no difference in response to both ESAs, 66.7% of male and 69.4% of female patients in group A corrected of anemia, while 62.1% of male and 57.1% of female patients in group B corrected of anemia, which represents no significant difference.
Study limitations
The limitations of this study were involvement of a single center and the small number of participants. The two groups were matched for several important confounders; however, other residual confounders, like the presence of inflammation, occult blood loss, missed dose injection, and others, could be there and not matched between the two groups.
Recommendations
If administration intervals could be successfully extended beyond once monthly for all patients, the resulting time savings could enable health care providers to spend more time focusing on other aspects of CKD management, including patient education, and to address other modifiable risk factors, such as hypertension and mineral balance.
Conclusions
Treatment with methoxy polyethylene glycolepoetin beta (Mircera) administered intravenously once monthly was superior to treatment with epoetinalfa (Eprex) administered subcutaneously three times weekly for maintaining haemoglobin concentrations in patients with chronic kidney disease on hemodialysis (p<0.0001). Safety findings were characteristic of the population under study and similar between the two treatment groups.
References
- Anía BJ, Suman VJ, Fairbanks VF, Rademacher DM, Melton LJ. Incidence of anemia in older people: an epidemiologic study in a well defined population. J Am Geriatr Soc. 1997; 45: 825-831. Ref: https://goo.gl/tCC1mN
- Levey AS, Eckardt KU, Tsukamoto Y, Levin A, Coresh J, et al. Definition and classification of chronic kidney disease: A position statement from kidney disease improving global outcomes (KDIGO). Kidney Int. 2005; 67: 2089-2100. Ref.: https://goo.gl/i6g9Qi
- Kidney Disease Improving Global Outcomes. KDIGO 2012 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease: Summary of Recommendation Statements. Kidney Int. 2013; 84: 5-14.
- Okada TS, Yanai MK, Takahashi S. The Kidney Early Evaluation Program (KEEP) of Japan: Results from the initial screening period. Kidney Int. 2010; 77: 17-23. Ref.: https://goo.gl/HDm4cr
- Goddard J, Turner A. Kidney and urinary tract disease, Davidson’s Principles and Practice of Medicine. 22th ed. NewYourk: Brian R. Walker Elsevier. 2014; 488.
- Bargman JM, Karl S, Joanne M. Chronic kidney disease, disorders of the kidney and urinary tract, Dennis L. Stephen L, (editors). Harrison principle of internal medicine. 19th edition. 2015; 1818-1826. Ref.: https://goo.gl/rC3dz8
- Macdougall I, Eckardt K. Novel strategies for stimulating erythropoiesis and potential new treatment for anemia. Lancet. 2006; 368: 947-953. Ref.: https://goo.gl/Fh2LNP
- Strippoli G, Manno C, Schena F, Craig JC. Haemoglobin and haematocrit targets for the anaemia of chronic renal disease. Cochrane Database Syst Rev. 2003; 1. Ref.: https://goo.gl/bs1DTk
- Collins A, Li S, Peter W, Ebben J, Roberts T, et al. Death, hospitalization, and economic associations among incident hemodialysis patients with hematocrit values of 36 to 39%. J Am Soc Nephrol. 2001; 12: 2465-2473. Ref.: https://goo.gl/y5FiDP
- Xue JL, St Peter WL, Ebben JP, Everson SE, Collins AJ. Anemia treatment in the pre-ESRD period and associated mortality in elderly patients. Am J Kidney Dis. 2002; 40: 1153-1161. Ref.: https://goo.gl/i8MsZD
- Besarab A, Bolton W, Browne J, Egrie JC, Nissenson AR, et al. The effects of normal as compared with low hematocrit values in patients with cardiac disease who are receiving hemodialysis and epoetin. N Engl J Med. 1998; 339: 584-590. Ref.: https://goo.gl/ozUF3K
- Mahon A, Docherty B. Renal anaemia: the patient experience. Edtna Erca J. 2004; 30: 34-37. Ref.: https://goo.gl/hFfRvG
- Annual data report: atlas of end-stage renal disease in the United States. United States Renal Data System. 2007.
- Carrera F, Disney A, Molina M. Extended dosing intervals with erythropoiesis-stimulating agents in chronic kidney disease: a review of clinical data. Nephrol Dial Transplant. 2007; 22. Ref.: https://goo.gl/H6atrt
- Macdougall I. C.E.R.A., a once-monthly ESA: is it living up to expectations? Port J Nephrol Hypert. 2009; 23: 219-223. Ref.: https://goo.gl/ARyS17
- McMurray J, Parfrey P, Adamson J, et al. KDIGO clinical practice guideline for anemia in chronic kidney disease. Kidney Int Suppl. 2012; 2: 1-335.
- Besarab A, Bolton WK, Browne JK, Egrie JC, Nissenson AR, et al. The effects of normal as compared with low hematocrit values in patients with cardiac disease who are receiving hemodialysis and epoetin. N Engl J Med. 1998; 339: 584-590. Ref.: https://goo.gl/WkHRnb
- Singh A, Szczech L, Tang KL, Barnhart H, Sapp S, et al. Correction of anemia with epoetin alfa in chronic kidney disease. N Engl J Med. 2006; 355: 2085-2098. Ref.: https://goo.gl/hsSAow
- Pfeffer M, Burdmann E, Chen CY, Cooper ME, de Zeeuw D, et al. A trial of darbepoetin alfa in type 2 diabetes and chronic kidney disease. N Engl J Med. 2009; 361: 2019-2032. Ref.: https://goo.gl/qNeqUG
- Fernando C, Charmaine EL, Angel de F, Francesco Locatelli, Johannes F.E. Mann, et al. Maintenance treatment of renal anaemia in haemodialysis patients with methoxy polyethylene glycol-epoetin beta versus darbepoetin alfa administered monthly: arandomized comparative trial. Nephrol Dial Transplant. 2010; 25: 4009-4017. Ref.: https://goo.gl/HYd8db
- Collins A, Brenner R, Ofman JJ, Chi EM, Stuccio-White N, et al. Epoetin alfa use in patients with ESRD. An analysis of recent US prescribing patterns and hemoglobin outcomes. Am J Kidney Dis. 2005; 46: 481-488. Ref.: https://goo.gl/vUscs9