Research Article
The pattern of blood pressure and renal function among children with Sickle Cell Anaemia presenting in a tertiary health institution in Nigeria

Adebukola Ajite1*, Ezra Ogundare1, Oludare Oluwayemi1, Oladele Olatunya1, Oluwasola Oke1, Kayode Tolorunju2 and Evelyn Omoniyi1
1Department of Paediatrics, Ekiti State University Teaching Hospital, Ado Ekiti, Nigeria
2Department of Chemical Pathology, Ekiti State Teaching Hospital, Ado Ekiti, Nigeria
*Address for Correspondence: Dr. Adebukola Ajite, Department of Paediatrics, Ekiti State University Teaching Hospital, PMB 5355, Ado Ekiti, Nigeria, Tel: +2348069712558; Email: bidemi_kayode@yahoo.com; adebukolaajite@yahoo.com
Dates: Submitted: 01 April 2019; Approved: 15 April 2019; Published: 16 April 2019
How to cite this article: Ajite A, Ogundare E, Oluwayemi O, Olatunya O, Oke O, et al. The pattern of blood pressure and renal function among children with Sickle Cell Anaemia presenting in a tertiary health institution in Nigeria. J Clini Nephrol. 2019; 3: 083-092. DOI: 10.29328/journal.jcn.1001031
Copyright License: © 2019 Ajite A, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
Background: In sickle cell anemia (SCA), compromise of the renal vasculature due to sickled red cells has been recognized.
Objectives: To assess the renal function and blood pressure pattern in children with sickle cell anaemia (SCA) presenting in a tertiary institution.
Method: A cross-sectional study of patients with sickle cell anaemia (SCA) over six months involving the use of questionnaires, general physical examination, blood pressure, investigations for haemoglobin genotype, urinalysis, serum creatinine, screening for hepatitis B and HIV.
Results: 51 children with SCA were seen. The prevalence of impaired renal function as defined by reduced eGFR <90mL/min/1.73m2 in this study was 27.5%, previous hospital admission and blood transfusion were associated with reduction in eGFR but blood pressure did not have significant correlation with the eGFR. The overall mean age at diagnosis of SCA was 4.09 ± 3.33 (years).
Conclusion: Impaired renal function is a major comorbid condition in children with SCA. In countries/locations where there is no newborn screening for sickle cell disease, diagnosis is delayed, thus detecting impaired renal function may be delayed, therefore the need for early detection and management is imperative.
Introduction
Sickle cell disease (SCD) is a clinical condition in which an individual inherits two abnormal haemoglobin (Hb) genes following mutation and at least one of which is Hemoglobin S (HbS) [1]. HbS is the result of a single base pair change, thymine for adenine, at the 6th codon of the β-globin gene [1]. This change encodes valine instead of glutamine in the 6th position [1,2]. Consequently, there is production of an unstable isoform which when slowly deoxygenated can polymerize leading to the production of sickle cell [3]. The most severe type is HbSS, otherwise called sickle cell anaemia (SCA) which occurs when both β-globin genes have the sickle cell mutation [1,2]. Sickle cells lack the fluidity of the normal erythrocytes and can impede capillary flow thereby leading to tissue ischaemia [3,4]. The kidney, being a highly vascular organ, is vulnerable to vaso-occlusive events. The haemodynamic changes that occur with chronic anaemia, renal hypoxia from recurrent vaso-occlusion and haemolysis-related endothelial dysfunction can lead to functional and structural changes presenting as haematuria, proteinuria, concentrating defect, renal insufficiency and hypertension which may progress to chronic kidney disease (CKD) [5-8].
Renal disease in patients with sickle cell anemia is a major comorbid condition that is seen in 15-18 % of all SCD patients, and is a cause of early death [5,9]. The presence of renal failure in sickle cell disease (SCD) ranges from 5 to 18% of the total population of SCD patients [9].
Blood pressure has been found to be lower in SCD patients when compared with their healthy counterparts with normal haemoglobin genotype and this has been attributed to the low systemic vascular resistance seen in them [10-15]. For the same reason, hypertension is uncommon in sickle cell disease patient, hypertension in them predisposes to greater risk of vaso-occlusive crisis and death [16]. Compromise of the renal vasculature due to sickled red cells has been associated with glomerular hypertrophy, glomerular hyperfiltration and consequent proteinuria [16]. Hemolysis in SCA may also contribute to the development of kidney disease due to the increase in levels of plasma free hemoglobin and subsequent hemoglobinuria. The free heme filtered through the glomerulus is directly cytotoxic to renal tubular epithelial cells and induces damaging inflammatory responses [17].
Objectives
This study was conducted to contribute to existing data on the pattern of blood pressure and renal function among children with sickle cell anaemia.
Study design and location
A cross-sectional study of patients diagnosed to have sickle cell anaemia who were attending the paediatric haematology clinic of Ekiti State University Teaching Hospital Ado Ekiti was done over a period of six months from June 2016 to December 2016 after obtaining an ethical clearance from the Ethical and research committee of the institution.
Data collection and sampling technique
Patients presenting at the paediatric haematology clinic during the duration of the study (six months) were serially recruited provided they met the inclusion criteria and after obtaining informed parental consent as well as assent from the patients, where applicable. Inclusion criteria included patients aged two to sixteen years who were diagnosed by haemoglobin electrophoresis to have sickle cell anaemia and presenting at the paediatric haematology clinic over a period of six months. Exclusion criteria included patients who had crises or were admitted at least two weeks prior to their scheduled clinic visit, patients on blood pressure lowering medication and those on steroids. Questionnaires were administered to consenting parents and subjects. Information requested for included the age, gender, age at diagnosis, regularity of clinic attendance, compliance with routine medication, number of hospital admissions in a year, common crisis noticed and previous blood transfusion. General physical examination, weight, height and blood pressure of the subjects were done alongside investigations such as haemoglobin genotype, urinalysis, serum creatinine, hepatitis B and HIV screening.
Blood pressure measurement
Blood pressure was determined using standard procedure according to the recommendation of the task force on high blood pressure in children and adolescent [17]. This was done by auscultation in the right arm after a 10-minute resting period using the mercury gravity sphygmomanometer with appropriate bladder cuff sizes. The onset of the first tapping sound (Korotkoff sound 1) was taken to indicate the systolic blood pressure (SBP) while the point of complete disappearance of the sound (Korotkoff sound 5) was taken to indicate the diastolic blood pressure (DBP). For each subject two measurements were taken after an initial blood pressure trial run to achieve subject’s confidence and composure. The average reading was then determined [18].
Urinalysis
Freshly voided urine was collected from every subject into a plain universal bottle and the UriScreen Combi 10 dipstick was used to assess urine protein semi-quantitatively, specific gravity of the urine as well as the presence of erythrocyte were also assessed. The test strip was dipped into the freshly voided urine for approximately 1 second, and then drawn across the edge of the container to remove the excess urine. After 30 seconds, the test strip was compared with the colour scale and the result was recorded immediately. Colour changes taking place after 2 minutes was regarded as of no significance. The amount of protein in the urine was assessed as negative, trace, 1+ (30 mg /dL), 2+ (100 mg/ dL), 3+ (500 mg/dL).
Patients with proteinuria had repeat urinalysis on three consecutive occasions over three months to ascertain those at risk of chronic kidney disease.
Estimated glomerular filtration rate
This was calculated by using the Schwartz formula [19], where the estimated glomerular filtration rate = kh/ serum creatinine in mg/dl (k is a constant 0.55 in children and adolescent girls and 0.77 in adolescent male, h is the height in cm) and serum creatinine was assessed using modified Jaffe’s method, type is endpoint, wavelength 520nm and the instrument is Spectioscan 60 DV, Biotech Engineering co.uk.
Data analysis
Data analysis was performed using both descriptive and comparative statistics. Descriptive statistics comprising of mean, standard deviation, percentages and proportions were used for the age, anthropometry, estimated glomerular filtration rate and blood pressure profile of the subjects.
The comparative statistics comprised of Chi-square test for categorical data, independent samples t-test and analysis of variance (ANOVA) for comparison of the mean of continuous dependent variables for categorical variables with three or more categories. Pearson’s correlation was also done.
Results
Fifty one children with sickle cell anaemia were studied over a period of six months with the age range of two to sixteen years. Thirty nine (76.5%) of them were male while twelve (23.5%) of them were female. All the subjects had homozygous haemoglobin SS. Serology test for hepatitis B, C and HIV were negative in all of them.
The biodata and anthropometric characteristics of the subjects
Age and sex distribution: The male to female ratio was 3.25:1 Out of the 51 subjects, 19 (37.3%) were aged 2-5years, 16 (84.2%) of them were males and 3 (15.8%) were females. Those aged 6-9years were 13 (25.5%), 10 (76.9%) of them were males and 3 (23.1%) were females. Those whose ages were ≥ 10 years were 19 (37.2%) and 13 (68.4%) of them were males and the remaining 6 (31.6%) were females.
Table 1 shows a comparison of the mean age at the time of the study, mean age at diagnosis of the disease condition, mean anthropometry and blood pressure profile based on the gender. There were no statistically significant differences in these parameters between genders. The overall mean age at diagnosis was 4.09 ± 3.33 (years), however, the mean age at diagnosis was earlier in male as compared to female. The mean values for blood pressure, mean arterial pressure and estimated glomerular filtration rate were all within normal range for each gender and there were no statistically significant difference between them.
| Table 1: Age, anthropometric, estimated glomerular filtration rate and blood pressure profile of the subjects. | ||||
| Age, anthropometric and blood pressure characteristics | Overall mean ± Standard deviation N = 51 | Male N = 39 |
Female N=12 |
p value |
| Age at onset of the study(years) | 7.92 ± 4.18 | 7.72 ± 4.32 | 8.58 ± 3.78 | 0.536 |
| Age at diagnosis (years) | 4.09 ± 3.33 | 3.75 ± 5.23 | 5.19 ± 3.56 | 0.193 |
| Height (cm) | 117.24 ± 22.56 | 116.56 ± 24.10 | 119.47 ± 17.34 | 0.700 |
| Weight (kg) | 23.37 ± 9.43 | 23.13 ± 9.66 | 24.12 ± 9.00 | 0.753 |
| Systolic blood pressure (mmHg) | 93.21 ± 10.96 | 94.51 ± 10.86 | 89.00 ± 10.67 | 0.129 |
| Diastolic blood pressure (mmHg) | 52.08 ± 7.55 | 52.56 ± 7.21 | 50.50 ± 8.70 | 0.413 |
| Mean arterial blood pressure (mmHg) | 65.79 ± 7.59 | 66.56 ± 7.17 | 63.33 ± 8.67 | 0.200 |
| Estimated glomerular filtration rate (ml/min/1.73m2) | 105.75 ± 35.66 | 105.12 ± 37.87 | 107.81 ± 28.61 | 0.822 |
Parents level of formal education, clinic attendance and routine medication
Among the subjects, 37 (72.5%) of the fathers had at least tertiary level of education, while 33 (64.5%) of the mothers had same. Majority of the patients 35 (69%) attended haematology clinic regularly, at least once in two months, however, the teenagers rarely came for follow up. The routine medication used by these subjects included; folic acid, multivitamin and Proguanil.
Blood pressure percentiles
Among the male subjects the systolic blood pressure percentiles recorded were; less 50th percentile [21(53.8%)], 50th percentile [2(5.1%)], >50th percentile to <90th percentile [13(33.3%)] and 90th percentile [3(7.7%)]. The diastolic blood pressure percentile recorded were; <50th percentile [19(48.7%)], 50th percentile [2(5.1%)], >50th -<90th percentile [17(43.6%)] and 90th percentile [1(2.6%)]. Among the female subjects 10(83.3%) had the systolic blood pressure percentiles less than 50th percentile while 2(16.7%) had theirs between >50th percentile to <90th percentile. The diastolic blood pressure percentiles recorded were; <50th percentile [9(75.0%)], 50th percentile [2(16.7%)] and >50th percentile to <90th percentile [1(8.3%)]. Majority of all the subjects had both diastolic and systolic blood pressure less than the 50th percentile and none of the subjects had blood pressure percentile above the 90th percentile for age and gender.
Serum Creatinine and estimated glomerular filtration rate (eGFR)
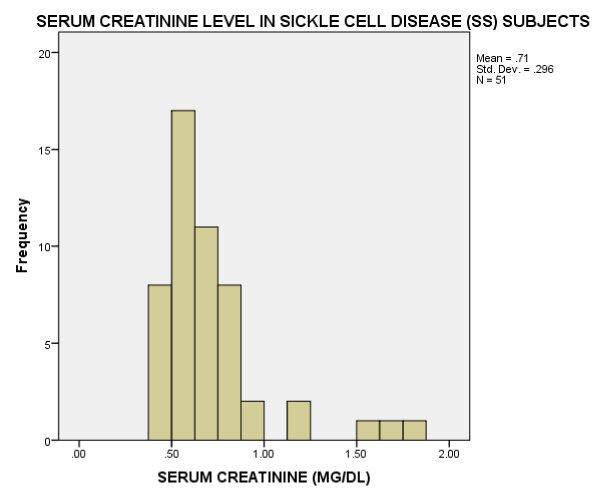
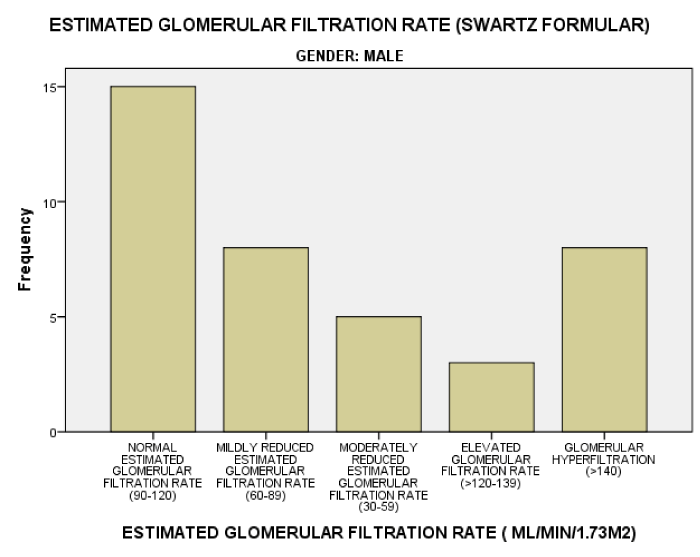
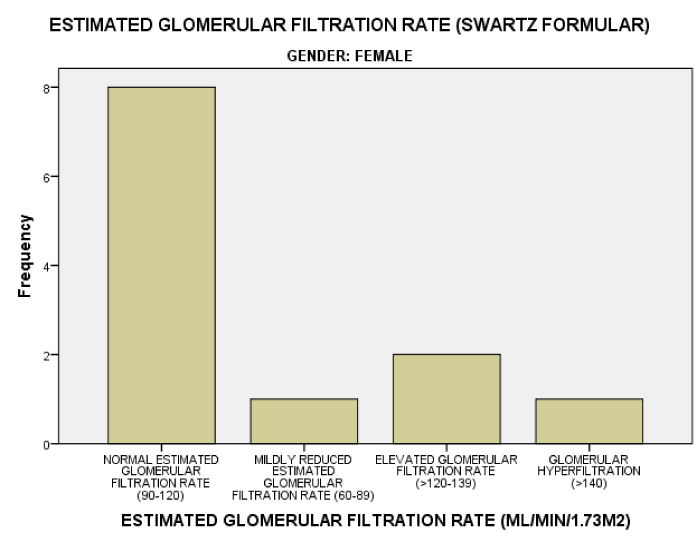
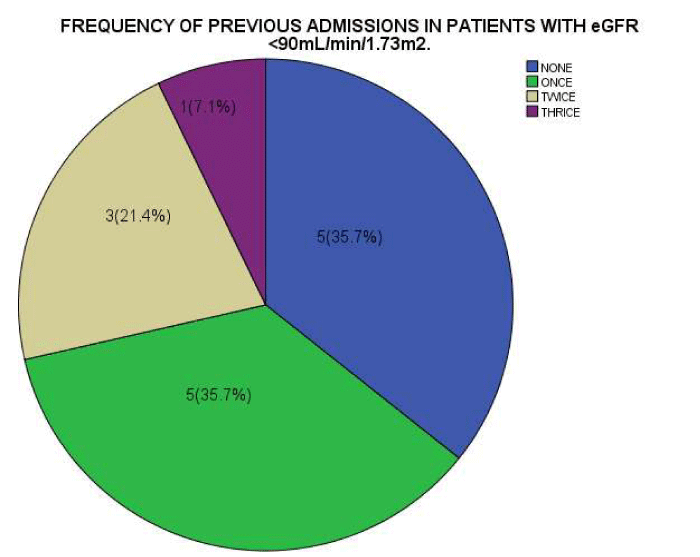
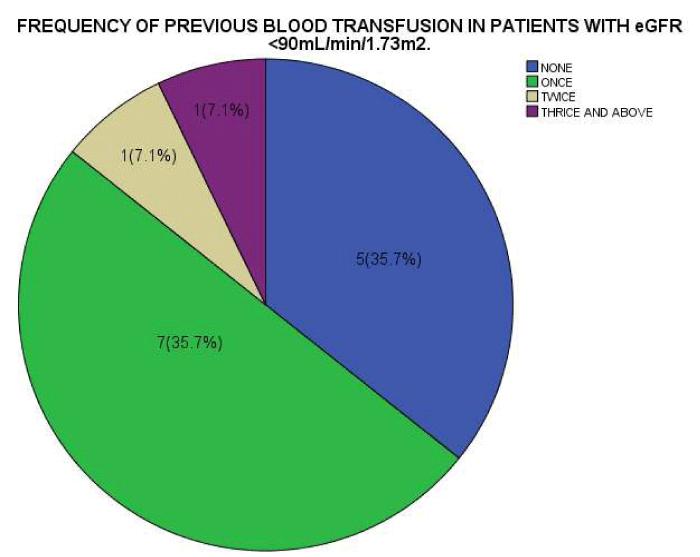
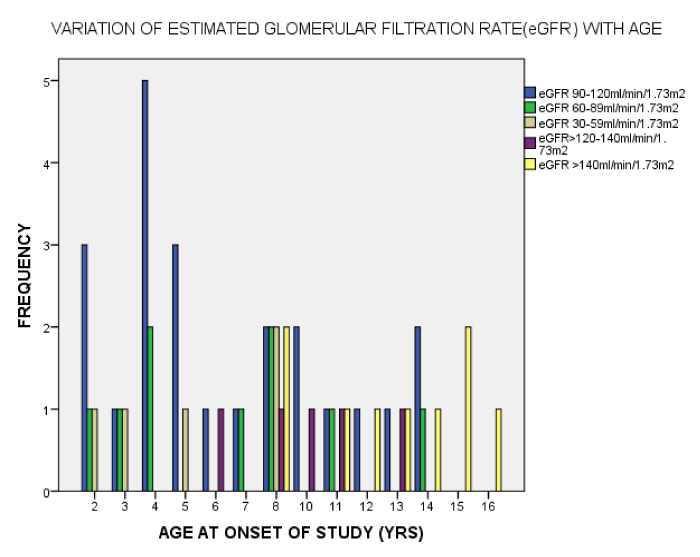
As seen in figure 1, the serum Creatinine levels were majorly less than 1mg/dl. The eGFR value for the subjects ranged from 32.2mL/min/1.73m2 to 197.0mL/min/1.73m2. The mean eGFR for the subjects was 105.75±35.66. In figure 2, the eGFR for the male subjects were divided into classes of normal eGFR (90-120ml/min/1.73m2), mildly reduced eGFR(60-89 ml/min/1.73m2), moderately reduced eGFR (30-59 ml/min/1.73m2), severely reduced eGFR (29-15 ml/min/1.73m2), end stage kidney disease(<15 ml/min/1.73m2), elevated eGFR (>120-139 ml/min/1.73m2) and glomerular hyperfiltration (>140 ml/min/1.73m2) (Tables 2,3). Eight (20.5%) male subjects had mildly reduced eGFR while 5(12.8%) had moderately reduced eGFR. Majority of the female subjects had eGFR above 90 ml/min/1.73m2 as seen in figure 3, only one female subject had mildly reduced eGFR (60- 89mL/min/1.73m2). The prevalence of reduced eGFR (<90ml/min/1.73m2) among the study population was 27.5% (14 subjects). These patients with reduced glomerular filtration cut across age 2-13years; more than half of them had previous hospital admission and blood transfusion as seen in figures 4 and 5 respectively. None of the subjects had eGFR <15mL/min/1.73m2. However, in figure 6, all the subjects with age ≥ 14 years had estimated glomerular filtration suggestive of glomerular hyperfiltration (eGFR ≥ 140mL/min/1.73m2).
| Table 2: Estimated glomerular filtration rate of patients and the presence of proteinuria | ||||
| PROTEINURIA | ||||
| NEGATIVE | 30MG/DL | 100MG/DL | ||
| ESTIMATED GFR VALUE | > 90mL/min/1.73m2 | 32 | 5 | 1 |
| 60-89mL/min/1.73m2 | 8 | 0 | 0 | |
| 30-59mL/min/1.73m2 | 5 | 0 | 0 | |
| Total | 45 | 5 | 1 | |
| Table 3: estimated glomerular filtration rate and the presence of blood in the urine. | |||
| BLOOD IN THE URINE | |||
| NEGATIVE | POSITIVE | ||
| ESTIMATED GFR VALUE | > 90mL/min/1.73m2 | 37 | 1 |
| 60-89mL/min/1.73m2 | 8 | 0 | |
| 30-59mL/min/1.73m2 | 5 | 0 | |
| Total | 50 | 1 | |
Proteinuria and haematuria
The prevalence of dipstick proteinuria among the subjects was 11.8% (6 subjects), the proteinuria was in the range of 30mg/dl -100mg/dl. The age range of these subjects with persistent proteinuria was 2-13years and 4(67%) of them had previous blood transfusion and all were male. The eGFR range of subjects with proteinuria was 94.8-169.0mL/min/1.73m2. Three of these subjects had eGFR ≥ 140mL/min/1.73m2. One subject (12year old male) had dipstick detected blood in the urine; the eGFR was also above 90mL/min/1.73m2.
Correlation of age at diagnosis, anthropometry, blood pressure and estimated glomerular filtration rate.
Using Pearson’s correlation, there was a significant positive correlation of the age at diagnosis of the disease condition with the height and the weight of the patients, the correlation was 0.678 and 0.536 respectively and both were significant at 0.01level with 99%degree of confidence. The estimated glomerular filtration rate was also shown to have a significant positive correlation with the weight and height, the correlation was 0.678 and 0.536 respectively and both were significant at 0.01level. Blood pressure parameters had no significant correlation with the estimated glomerular filtration rate.
Discusion
In the study, there was a male preponderance with M:F of 3.25:1. The age range of the subjects was 2-16 years, it was noticed that the population of the subjects was like a pyramid with a decline in the population as the age increased. This could be explained by the increase in mortality and poor survival of children with haemoglobinopathy in resource poor setting [20], such as the location of this study. The increase in mortality and poor survival may be attributed to ignorance of the disease condition, poverty, environmental factors like the malaria endemicity and delay in presentation in the hospital. The overall mean of age of the subjects at first diagnosis was 4.09 ± 3.33 years which depicted delay in diagnosis when compared with developed countries with the facility for newborn screening. This also suggests that the haplotype of sickle cell in these patients may be of less severity, making the patients to present to the hospital with symptoms at older age since facility for newborn screening is not widely available yet. There is also the possibility that the most severe cases had died before presenting at the hospital for routine care.
The blood pressure pattern showed that both systolic and diastolic blood pressure were below 50th percentile for most of the patients across genders. This is in agreement with the documentation of low blood pressure in sickle cell disease patients which has been attributed to low systemic vascular resistance seen in them [3]. The serum creatinine levels were majorly less than 1mg/dl in the recruited subjects. Reduced muscle mass as compared with normal healthy individuals may be one of the contributing factors. Similar finding was reported in a study among adults with sickle cell disease [21].
The prevalence of dipstick positive proteinuria in this study was 11.8%, this is lower than the prevalence of 20.0% recorded in adult Nigerian patients with SCA by Aneke et al. [22]. This disparity can be explained by the difference in the ages of the study population. Out of the six subjects with persistent proteinuria, 5(83.3%) of them were above the age of 5years, the tendency to have persistent proteinuria seemed to increase with age. Age has been described as the most potent modifying factor of sickle nephropathy of which persistent proteinuria is a feature and progression to overt CKD has been noticed in early adulthood [23].
In this study, the six patients with proteinuria had their eGFR in the range of 94.8-169.0mL/min/1.73m2 and half of them had eGFR above 140ml/min/1.73m2 suggestive of glomerular hyperfiltration. A previous study [24] reported similar finding that higher rates for developing microalbuminuria were seen in SCA patients with glomerular hyperfiltration and the same study also noted that the prevalence of hyperfiltration decreased with age [24]. These subjects may be in the early phase of kidney damage with compensatory increase in glomerular filtration which may be responsible for the enhanced glomerular passage of albumin and larger proteins that has been previously described in SCD [3]. Age related progression to chronic kidney disease and decrease in renal function may be implicated in the reduction of the prevalence of hyperfiltration with age as seen in the study of adults with SCA [21].
The prevalence of impaired renal function as defined by reduced estimated glomerular filtration rate <90mL/min/1.73m2 in this study was 27.5%. The subjects with reduced glomerular filtration rate cut across ages 2-13years and had at least an episode of previous hospital admission and blood transfusion. This suggests that renal impairment is common in sickle cell anaemia and that sickle cell crisis which is severe enough to warrant hospital admission and blood transfusion may be a contributing factor. Ongoing hemolysis and vaso-occlusive injury have been reported to likely contribute to continuing renal injury [17,23]. Among patients with sickle cell disease, decreased kidney function has been reported in 5 to 30 percent [21,25,26], which is close to our finding in this study. A higher prevalence of 67.6% was recorded by Yusuf et al. [27], in his study of adults with SCA in Zaria, Nigeria using similar cut off point of eGFR of <90 ml/min/1.73m2. Previous scholars have suggested that as more individuals with SCD are reaching the fourth to sixth decade of life, the prevalence and risk of CKD is likely to increase as well [23], this may explain the higher prevalence of decrease kidney function seen in adults with SCD.
The range of eGFR in normal children aged 2-12years was put at 89-165mL/min/1.73m2 by Heilben et al. in 1991 [28], however in this study among children with sickle cell anaemia, the range was 32.2mL/min/1.73m2 -197.0mL/min/1.73m2. Worthy of note is the fact that all subjects above the age of 14years had the estimated glomerular rate above 140ml/min/1.73m2. An earlier study by Olowu et al. [29], among children with sickle cell disease patient aged 5-13years showed that Nigerian children with homozygous sickle cell anaemia who are in steady states have normal glomerular filtration rate while studies in adult SCD showed reduced glomerular filtration rate [19,20]. The disparity in the outcome of these various studies may be due to the fact that the changes in the glomerular filtration rate could be a spectrum; with the earlier ages 5-13years having predominantly normal glomerular filtration rate [23], ages > 14years having glomerular hyperfiltration as a feature of compensation in early kidney disease as seen in this study and the adults SCD patients having reduced glomerular filtration rate due to age related renal decompensation [21,25]. A similar continuum of hyposthenuria in children to progression to end-stage renal disease (ESRD) in adults has been described by other authors [23]. Although in the early ages the eGFR values were predominantly normal in this study, evidence of glomerular hyperfiltration as suggested by eGFR >140ml/min/1.73m2 was seen across all the ages from 2-16 years. Similarly, hyperfiltration has been seen as early as 9–19 months of age according to the Pediatric Hydroxyurea Phase III Clinical Trial (BABY HUG) study [30]. The compromise of the renal vasculature due to sickled red cells has been associated with glomerular hyperfiltration, proteinuria and glomerular hypertrophy. However, there is need for further elaborative study to unravel factors implicated in the outset of glomerular hyperfiltration in sickle cell anaemia.
There was no correlation between the estimated glomerular filtration rate and the blood pressure of the subjects in this study; this is similar to previous observation that in contrast to other glomerulopathies, the development of systemic hypertension is uncommon in HbSS subjects with renal insufficiency [21].
Conclusion
The prevalence of renal insufficiency as demonstrated by estimated glomerular filtration rate of <90mL/min/1.73m2 was 27.5%. Previous hospital admission and blood transfusion were associated with reduction in eGFR but blood pressure did not have significant correlation with the eGFR. There is delay in diagnosis of SCA and the prevalence of persistent proteinuria and glomerular hyper filtration was noticed to increase with the age of the subjects. Therefore, there is a need for early detection and management of SCD to forestall recurrent vasoocclusive crises and end organ (kidney) damage. However, in the absence of newborn screening for SCD in some countries/locations leading to delay in diagnosis, early detection of renal function is critical. Routinely, renal function in sickle cell disease patients should be monitored as some might actually have impaired renal function with a need to adjust dose of medication, ensure prompt intervention and follow up.
References
- Michael R, De Baun Elliott Vichinsky. Hemoglobinopathies: Nelson Textbook of Pediatrics, RE Berman, RM Kliegman, HB Jenson, BF Stanton. 18th ed. Philadephia, 2007.
- Adekile AD, Adeodu O. Hemoglobinopathies. Paediatrics and child health in tropical region. 2nd ed 373-390.
- Guasch A, Cua M, Mitch WE. Early detection and the course of glomerular injury in patients with sickle cell anemia. Kidney Int. 1996; 49: 786-791. Ref.: https://tinyurl.com/y3fz4nyh
- Guasch A, Cua M, You W, Mitch WE. Sickle cell anaemia causes a distinct pattern of glomerular dysfunction. Kidney Int. 1997; 51: 826-833. Ref.: https://tinyurl.com/y3pd2mb5
- Serjeant GR, Serjeant BE. Sickle Cell Disease, 3rd ed. edn. Oxford, United Kingdom: Oxford University Press. 2001; Ref.: https://tinyurl.com/y6qvjrdn
- Kadiri S. Chronic kidney disease: Sickle cell nephropathy as a likely cause. Annals of Ibadan Postgraduate Med. 2006; 4: 7-10.
- Day TG, Drasar ER, Fulford T, Sharpe CC, Thein SL. Association between hemolysis and albuminuria in adults with sickle cell anemia. Haematologica. 2012; 97: 201–205. Ref.: https://tinyurl.com/y59xahlj
- Haymann JP, Stankovic K, Levy P, Avellino V, Tharaux PL, et al. Glomerular hyperfiltration in adult sickle cell anemia: a frequent hemolysis associated feature. Clin J Am Soc Nephrol. 2010; 5: 756–761. Ref.: https://tinyurl.com/y27xrxyc
- Saborio P, Scheinman JI. Sickle cell nephropathy. J Am Soc Nephrol. 1999; 10: 187–192. Ref.: https://tinyurl.com/y6jso6o6
- Guasch A. Primer on kidney disease, 4th edition. National Kidney Foundation. Sickle cell Nephropathy. 351-355.
- Johnson CS. Arterial blood pressure and hypervicosity in sickle cell disease. Hematol Oncol Clin North Am. 2005; 19: 827-837. Ref.: https://tinyurl.com/y3h3c6tb
- Johnson CS, Giorgio AJ. Arterial blood pressure in adults with sickle cell disease. Arch Intern Med. 1981; 141: 891-893. Ref.: https://tinyurl.com/yygzykn2
- Grell GA, Alleyne GA, Serjeant GR. Blood pressure in adults with homozygous sickle cell disease. Lancet. 1981; 2: 1166. Ref.: https://tinyurl.com/y5jl3w5s
- Pegelow CH, Colangelo L, Steinberg M, Wright EC, Smith J, et al. Natural history of blood pressure in sickle cell disease: risks for stroke and death associated with relative hypertension in sickle cell anemia. Am J Med. 1997; 102: 171-177. Ref.: https://www.ncbi.nlm.nih.gov/pubmed/9217567
- Karayaylalí I, Onal M, Yíldízer K, Seyrek N, Paydas S, et al. Low blood pressure, decreased incidence of hypertension, and renal cardiac, and autonomic nervous system functions in patients with sickle cell syndromes. Nephron. 2002; 91: 535-537. Ref.: https://tinyurl.com/y5vgcaep
- Hsien HC, Carvalhaes JTA, Braga JAP. Blood pressure in Children with sickle cell disease. Revista Paulista de Pedatria. 2012; 30: Ref.: https://tinyurl.com/y5ldc2sw
- De Miguel C, Speed JS, Kasztan M, Gohar EY, Pollock DM. Endothelin-1 and the kidney: new perspectives and recent findings. Curr Opin Nephrol Hypertens. 2016; 25: 35–41. Ref.: https://tinyurl.com/y64e3kv3
- Falkner B, Daniels SR, Flynn JT, Gidding S, Green LA, et al. The Fourth Report on the Diagnosis, Evaluation and Treatment of High Blood Pressure in Children and Adolescents. Pediatrics. 2004; 114: 555-576. Ref.: https://tinyurl.com/y39b9fs6
- Schwatz GJ, Brion CP, Spitzer A. The use of plasma creatinine concentration for estimating GFR in infants, children and adolescents. Pediatr Clin North Am. 1987; 34: 571-590. Ref.: https://tinyurl.com/yyah7644
- Epidemiology of Hb Disorders WHO. 2008; Weatherall DJ, Blood. 2010.
- Guasch A, Navarrete J, Nass K, Zayas CF. Glomerular involvement in adults with sickle cell hemoglobinopathies: Prevalence and clinical correlates of progressive renal failure. J Am Soc Nephrol. 2006; 17: 2228-2235. Ref.: https://tinyurl.com/y4xwh3fa
- Aneke JC, Adegoke AO, Oyekunle AA, Osho PO, Sanusi AA, et al. Degrees of Kidney Disease in Nigerian Adults with Sickle-Cell Disease. Med Princ Pract 2014; 23: 271-274. Ref.: https://tinyurl.com/y3jt2hzp
- Rakhi P, Naik, Vimal K. Derebail. The spectrum of sickle hemoglobin-related nephropathy: from sickle cell disease to sickle trait. Expert Rev Hematol. 2007; 10: 12: 1087-1094. Ref.: https://tinyurl.com/y5lpkxoz
- Vazquez B, Shah B, Zhang X, Lash JP, Gordeuk VR, et al. Hyperfiltration is associated with the development of microalbuminuria in patients with sickle cell anemia. Am J Hematol. 2014; 89: 1156–1157. Ref.: https://tinyurl.com/y22p6p2j
- Sklar AH, Campbell H, Caruana RJ, Lightfoot BO, Gaier JG, et al. A population study of renal function in sickle cell anemia. Int J Artif Organs. 1990; 13: 231. Ref.: https://tinyurl.com/y3gpvevp
- Falk RJ, Scheinman J, Phillips G, Orringer E, Johnson A, et al. Prevalence and pathologic features of sickle cell nephropathy and response to inhibition of angiotensin-converting enzyme. N Engl J Med. 1992; 326: 910-915. Ref.: https://tinyurl.com/yyrr8nvs
- Yusuf R, Hassan A, Ibrahim I N, Babadoko AA, Ibinaiye PO. Assessment of kidney function in sickle cell anemia patients in Zaria, Nigeria. Sahel Med J. 2017; 20: 21-25. Ref.: https://tinyurl.com/yxw9jw2j
- Heilbron DC, Holliday MA, al-Dahwi A, Kogan BA. Expressing glomerular filtration rate in children. Pediatr Nephrol.1991; 5: 5-11. Ref.: https://tinyurl.com/y5x7v25v
- Olowu WA, Taiwo O, Oyelami A, Durosinmi MA, Adeodu OO, et al. Glomerular filtration rate in Nigerian children with homozygous sickle cell disease. Niger J Med. 2002; 11: 23-25. Ref.: https://tinyurl.com/y56jut3l
- Ware RE, Rees RC, Sarnaik SA, Iyer RV, Alvarez OA, et al. BABY HUG Investigators. Renal function in infants with sickle cell anemia: baseline data from the BABY HUG trial. J Pediatr. 2010; 156: 66–70. Ref.: https://tinyurl.com/y5p3xr3z