Research Article
High water intake in preventing the risk of Uric Acid Nephrolithiasis: A systematic review and meta-analysis

Zhijian Lin1, John C Lieske2*, Yue Li3 and Muthuvel Jayachandran4
1Beijing University of Chinese Medicine School of Chinese Pharmacy, Beijing, China
2Department of Nephrology and Hypertension, Mayo Clinic, Rochester, MN, USA
3University of Minnesota School of Public Health, Minneapolis, MN, USA
4Department of Physiology and Biomedical Engineering, Mayo Clinic, Rochester, MN, USA
*Address for Correspondence: John C Lieske, Department of Nephrology and Hypertension, Mayo Clinic, Rochester, MN, USA, Tel: +1-5073194234; Email: Lieske.John@mayo.edu
Dates: Submitted: 27 June 2019; Approved: 11 July 2019; Published: 12 July 2019
How to cite this article: Lin Z, Lieske JC, Li Y, Jayachandran M. High water intake in preventing the risk of Uric Acid Nephrolithiasis: A systematic review and meta-analysis. J Clini Nephrol. 2019; 3: 126-142. DOI: 10.29328/journal.jcn.1001038
Copyright License: © 2019 Lin Z, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
Background: Hyperuricosuria, persistently low urinary pH, and low urinary volume are the main risk factors of uric acid nephrolithiasis. Epidemiologic studies suggest that high water intake is protective against the occurrence of symptomatic kidney stone events of all types. The objective of this systematic review and meta-analysis were to evaluate the effectiveness of increased water intake to prevent symptomatic uric acid kidney stone events.
Methods: Seventeen studies were identified for the meta-analysis. Analysis of Q and I2% statistics revealed that a high heterogeneity in 16 studies, thus, random effects model was used. Protective associations were identified for high water intake individuals (SMD=0.52 L; 95% CI: 0.19, 0.84; p=0.002); a significantly decreased relative super saturation of uric acid versus controls (SMD=-1.15; 95% CI: -2.00, -0.30; p=0.008). Risk factors including urinary uric acid excretion and pH were not significantly related to high water intake (SMD=7.32mg/d, 95% CI: -52.27, 66.91; p=0.81), (SMD=0.14; 95% CI: -0.02, 0.31; p=0.09), respectively. Further subgroup analyses revealed that urinary uric acid excretion was significantly decreased in healthy individuals (SMD=-36.23 mg/d, 95% CI: -65.14, -7.31; p=0.001) compared to stone formers (SMD=27.41 mg/d, 95% CI: -33.18, 88.01; p=0.38); urinary uric acid excretion was significantly decreased in routine water intake groups (SMD=-61.49 mg/d, 95% CI: -120.74, 12.24; p=0.04) compared to mineral water intake groups (SMD=44.50 mg/d, 95% CI: -18.30, 107.29; p=0.16); urinary pH was significantly higher in mineral water groups (SMD=0.13, 95% CI: 0.01, 0.46; p=0.04) compared to regular water groups (SMD=-0.00, 95% CI: -0.13, 0.13; p=0.98).
Results: A total of 129 patients had 150 internal jugular catheter insertions. The mean age was 51.4±15.2 years with male to female ratio of 1.5:1. All the patients had chronic kidney disease; about 80% had tunneled IJC and 96.9% of the catheters were inserted in the right internal jugular vein. Immediate complications were recorded in 10% and late complications in 34.9% of the procedures. The immediate complications were kinking of guide wire (2%), arterial puncture (1.3%) and difficulty in locating the internal jugular vein (1.3%) or tunneling (1.3%). The late complications were infection (12.8%), poor blood flow (9.2%), bleeding (5.5%) and spontaneous removal of the catheter (5.5%). There was no statistical significant difference in both immediate and late complication with age and sex.
Conclusion: This meta-analysis identified evidence that urinary uric acid excretion, volume, pH and relative supersaturation of uric acid can be altered with high water intake intervention, reducing the risk of uric acid kidney stones.
Introduction
Uric acid nephrolithiasis (UAN) refers to the formation of urinary stones containing uric acid or urate salts. UAN constitutes around 10% of kidney stones. The incidence of UAN has constantly increased over recent years [1-3]. The United States National Health and Nutrition Examination Surveys II and III reported that kidney stone prevalence increased from 3.8% in the year 1976 to 5.2% in the year 1994 in most developed countries [4]. There are global geographical diversities in the incidence of UAN. The worldwide incidence ranges from 5% to 40% [3]. The prevalence of UAN was reported to be 15-16% in Japanese and Chinese descendants in San Francisco, 28% in Pakistan, 22% in Israel%. Approximately 13% of men and 7% of women will develop a kidney stone during their lifetime. UAN prevalence is higher in Middle Eastern and Hmong immigrant populations in the US [3,5,6]. The pathogenesis of UAN remains unclear. Three significant urinary abnormalities have been described as main etiological risk factors in the pathogenesis of UAN including hyperuricosuria, persistently low urinary pH, and low urinary volume [7]. From the viewpoint of treatment and prevention, hydration status, urine output and uric acid concentration are important factors that affect renal handling of uric acid [3]. Medical dissolution treatments including urine dilution and alkalization can be effective in many UA stone formers [8]. Indeed, increased fluid intake has been universally advocated as a prevention strategy for all types of kidney stones [9,10]. Some intervention studies specifically promote water, while some include other beverages that largely contain free water [10,11]. Increased water intake may help prevent the formation of UAN by diluting urinary uric acid concentration, and by flushing away uric acid micro-crystals [12]. Mineral waters may also decrease urine pH. Some studies support the efficacy of water therapy to prevent and reduce the recurrence of all forms of kidney stones lumped together, presumably since high water intake increases urine flow rates, lowers the super saturation for stone forming salts [10-13]. From that perspective, we hypothesized that UAN could be prevented by increasing urine volumes and flows via increased water intake [8-10].
Recently both the American College of Physicians (ACP) clinical guideline and American Urological Association (AUA) clinical guideline recommend sufficient water intake to prevent recurrent kidney stones [14,15]. However, the association between fluid intake and kidney stone risk is not entirely clear. Several studies suggest that sufficient fluid intake is an effective strategy to prevent kidney stones [12,16-18]. Three meta-analyses concluded that water intake was associated with reduced kidney stone risk and long-term risk of recurrence [8,13,18]. However, some studies contain conflicting results [19,20]. Further, neither the clinical guidelines nor the mate-analysis address the relationship between high water intake and prevention of UAN. Thus the objectives of this systematic review and meta-analysis were to define the quantitative relationship between high water intake and the UAN risk.
Materials and Methods
This systematic review and meta-analysis were performed using published studies including interventional and cross over studies.
Eligibility criteria
We included both healthy non stone formers and patients with a urolithiasis history in our selection criteria. Intervention study subjects must have been prescribed high water intake (any kind of water). Study designs could be interventional or prospective observational and included comparison to baseline or control untreated cohorts. Studies with urine profiles (as opposed to stone events as the outcome) were included. All had full text articles available. We did not limit based upon year or published language.
Search strategy
The literature search was conducted in PubMed, Science Direct, Cochrane library, and two Chinese databases (China National Knowledge Infrastructure and Wang fang Database) through November 2018. A manual search of the article references and relevant reviews was also completed. The keywords used for the search were as follows: “uric acid”, urate, kidney, renal, urinary, stone*, crystal*, nucleat*, *lithiasis, grow*, form, formation, forming, formed, precipitat*, solub*, water, coffee, tea, soda, fluid*, liquid*. The terms “humans” or “patient” was used to limit search results.
Study inclusion and exclusion criteria
Studies were included in the meta-analysis if they: were based on a cohort, case–control, or cross over design published as original studies; contained exposure information water intake (water, coffee, tea, soda); evaluated the effects of high water intake on urinary risk factors for UAN; provided data on 24 hour urinary uric acid, urinary pH, urine volume, relative super saturation of uric acid, and other risk factors related to UAN. Additionally, studies must have included a reference group composed of subjects on a usual water intake (control or baseline). We excluded literature review, grey literature, and conference abstracts.
Study selection and data extraction
All studies were organized and screened for duplication using EndNote X8 software. Duplication-free articles underwent title and abstract examination using predetermined inclusion and exclusion criteria described above. Study selection was performed by two investigators independently. Opinion discrepancies were resolved by discussion and ultimate consensus. All studies which fulfilled the inclusion and exclusion criteria underwent full text review. A standardized data collection form was used to extract the following information, if available: name of first author, year of publication, country of origin, title of article, study design, sample size, year of study, subjects specific condition, definition of high fluid intake (If there was no information on water serving size in an article, an assumption was made that a serving size of 1 cup or glass was equal to 150 mL), control intervention or baseline. The outcome parameters were chosen on the basis of the hypothesis that three main risk factors of UAN were hyperuricosuria, persistently low urinary pH, and low urinary volume. We extracted urine profile data as follows: 24 hour urinary uric acid, urinary pH, urine volume, relative supersaturating of uric acid. Measurement units used in this study were mg/day for urinary uric acid level, L/day for urine volume, and % for relative supersaturating of uric acid. All data are continuous variables, and numbers were extracted as mean and standard deviation.
Statistical analysis
Mean and standard deviation were combined by Review Manager 5.3.5 software (The Cochrane Collaboration, Oxford, UK). The degree of heterogeneity was assessed calculating the Cochran’ Q statistic with I2 and P heterogeneity. An I2 of 0%–25%, suggested no heterogeneity; 26%–50% represented low heterogeneity; 51%–75% represented moderate heterogeneity; and 75%–100% represented high heterogeneity [21,22]. The presence of publication bias was assessed by funnel plots [21]. Outcomes of 24 hour urinary uric acid, urinary pH, urine volume, and relative super saturation of uric acid were pooled under random-effects models and SMD as the effect size. If outcomes were observed in multiple follow-up times, we combined the mean and standard deviation, and pooled within-trial differences in SMDs [23]. Sensitivity analyses was used to ensure that no individual study was entirely responsible for the combined results, and this procedure was used to determine which study was the main source of heterogeneity [24]. We performed sensitivity analyses by removing each individual study in turn from the total and re-analyzing the remainder. We also performed subgroup analyses to detect possible sources of heterogeneity and potential difference among subgroups, by grouping the condition of subjects (healthy subjects or stone formers) and the type of water intake (regular water or mineral water). All P-values were 2-sided.
Results
Search results
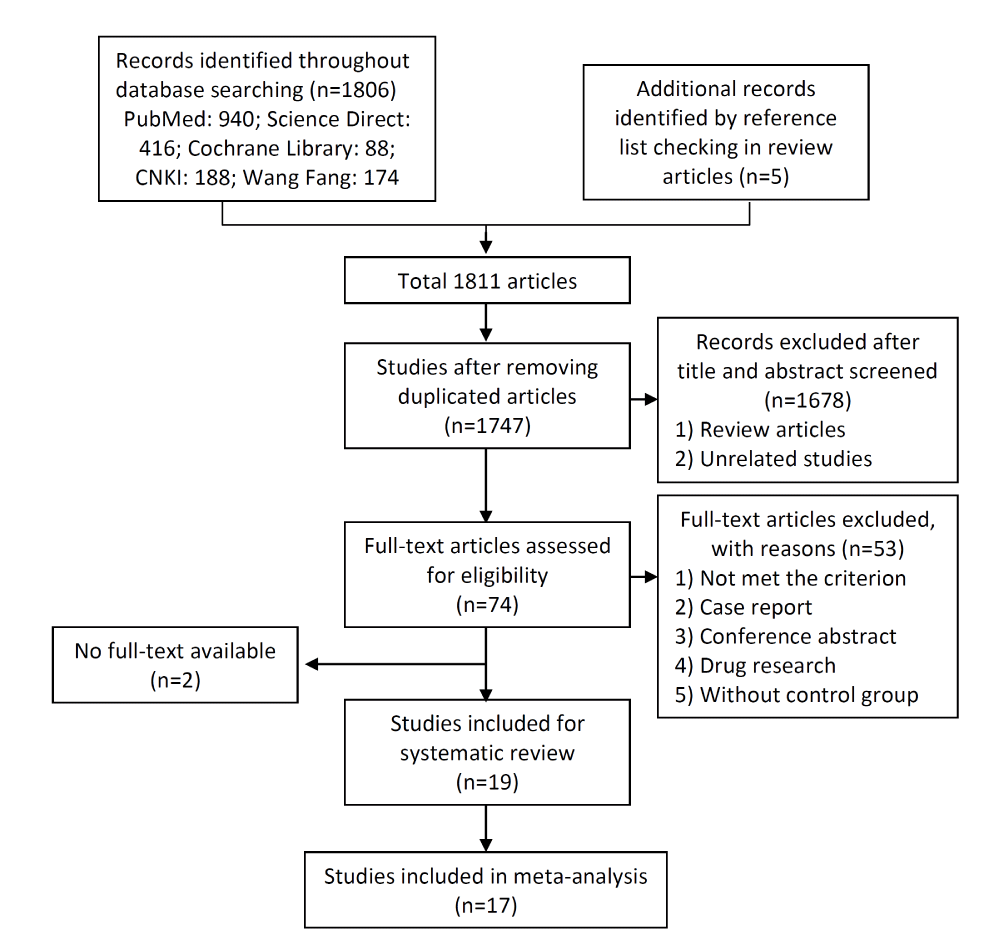
The search strategy identified 1811 potentially relevant articles. After removing duplicates, 1747 individual publications were screened by title and abstract, after removing review articles and not relevant studies. A total of 74 full manuscripts were identified for detailed evaluation. Fifty-three articles were excluded, including 2 articles with no full-text available. Nineteen articles were consistent with our selection criteria. Two of these 19 did not have sufficient quantitative analysis; therefore 17 studies remained for the full meta-analysis. A flow diagram illustrating study selection is shown in figure 1.
Characteristics of included articles
Nineteen studies were consistent with our selection criteria. Two of the 19 studies had to be excluded from quantitative analysis because of the following reasons: (1) Milewski JB et al. only published mean values as their outcome measurement, without standard deviation and p values [25]; and (2) Neimark AI et al. reported implausible data, since 24h urinary uric acid amount changed from 0.8±2.4 to 8.3±2.23 mmol/d after the treatment of Serebrianyi Kliuch mineral water [26]. Three of the remaining 17 studies were conducted in USA [27-29], two in China [30,31], two in Germany [32,33], one in Iran [34], four in Italy [12,35-37], one in Poland [25], three in Russia [26,38,39], two in Saudi Arabia [40,41], one in South Africa [42]. Among the studies two were randomized controlled trial. This systematic review retrieved a total number of 895 participants in the articles included; number of participants per study ranged from 6 to 100. The 2 RCT studies enrolled 399 participants with a follow-up period of 14 days [35] and 5 years [12]. Six studies included only healthy subjects, eleven studies included kidney stone formers, and two studies included both healthy subjects and patients. Only one study included children (n=3), the remaining including only adult participants [39]. Water intervention duration ranged from 2 days to 5 years. Characteristics of the studies included are shown in table 1.
| Table 1: Characteristic of included studies. [ordered by study ID]. | ||||||||
| Study author and year | Country | Type of study |
Subject condition | Water intake Intervention | n | Baseline or control | n | Main outcomes |
| Study included in qualitative and quantitative synthesis | ||||||||
| Abdulwahab A. Noorwali, et al (1988) | Saudi Arabia | Self-controlled study | Healthy adults (4 male, 3 female) | Drink 2 L of tea using one, two, or four bags for one week. Note: Tea bags (Lipton) were immersed in 2 L of boiling water and left without any mixing for five minutes. The tea bags were discarded, and the tea consumed throughout the day |
7 | Baseline: measured during the pre-phase | 7 | 24h urine volume Note: combining data from one, two, and four tea bags groups during overall analyses |
| Corey M. Passman, et al (2009) | America | Self-controlled study | Nonstone-forming healthy adult (2 male, 4 female) | Drink Le Bleu bottled water, volumes intake based on the volunteers’ lean body weight for 5 days | 6 | Baseline: measured at self-selected diet phase | 6 | 24h urinary uric acid, pH, urine volume |
| Danielle D. Sweeney, et al (2009) | America | Self-controlled study | Healthy subjects (5 male, 7 female) and 12 hypercalciuric stone formers (2 male, 10 female) |
All subjects ingested 2 L water daily for 7 days | 24 | Baseline: measured during the pre-phase | 24 | 24h urinary uric acid, pH, urine volume; relative supersaturation of uric acid Note: combining data from healthy and stone former groups during overall analyses |
| Di Silverio, F, et al (1994) | Italy | Randomized controlled trial | Patients with kidney stones (118 male, 82 female) | Drink more than 1.5 L of Fiuggi water daily for 7 and 14 days | 100 | Drink less than 1500 mL of Fiuggi water daily for 7 and 14 days | 100 | 24h urinary uric acid, pH Note: combining data from 7 and 14 days groups during overall analyses |
| Dzeranov NK, et al (2000) | Russia | Self-controlled study | Patients with urolithiasis (23 male, 29 female) | Drink a low mineral content water TIB-2 3 times a day in a dose 200 mL 30-45 minutes before meal for 12 days | 52 | Baseline: measured during the pre-phase | 52 | 24h urinary uric acid, urine volume Note: combining data from follow-up 3-5 day and 10-12 day during overall analyses |
| Fahad A. Alyami, et al (2011) | Saudi Arabia | Self-controlled study | Healthy volunteers | Drink at least 1.2 L daily of bottled water for 2 weeks | 20 | Baseline: measured during the pre-phase | 20 | 24h urinary uric acid, pH, urine volume |
| Kang Chen, et al (2018) | China | Self-controlled study | Healthy males with no previous history of urolithiasis or other renal disorders | Drink three cups of prepared 2g of green leaf tea for 7 consecutive days (about 600-800 mL). Following a washout period of 3 weeks, drink 4 g of green leaf tea for 7 consecutive days. Note: tea leafs were immersed in 200 mL of hot distilled water during 2 min before consumption, tea sample pH 5.68 |
12 | Baseline: measured during the pre-green tea intake | 12 | 24h urinary uric acid, pH, urine volume Note: combining data from 2g group and 4g group during overall analyses |
| LORIS BORGHI, et al (1996) | Italy | Randomized controlled trial | 220 patients with kidney stones, 21 dropouts during follow-up period | High water (not too mineralized) intake, which would give a urine volume that was equal to or greater than 2 L daily for 5 years | 99 | Did not provide for any high water treatment | 100 | 24h urine volume and relative supersaturation of uric acid Note: combining data from follow-up 5 years during overall analyses |
| Majid Mirzazadeh, et al (2012) | Iran | Self-controlled study | 14 patients with Calcium kidney stones; 15 patients without kidney stones, who were otherwise healthy patients undergoing urethroplasty |
Drink mineral water with low hardness 110 mg/L, Tehran tap water with moderate hardness 180 mg/L, and mineral water with high hardness 280 mg/L; at a dose of 120 mL of water per 100kcal/d (about 1.8-2.2 L) for two days. Note: water with low mineral hardness pH 6.5±0.20; Tehran tap water pH 7.2±0.45; mineral water with high hardness pH 6.9±0.95 |
29 | Baseline: measured at self-selected diet phase | 29 | 24h urinary uric acid, pH, urine volume Note: combining data from different hardness mineral water groups during overall analyses |
| Martino MARANGELLA, et al (1996) | Italy | Self-controlled study | Patients with idiopathic calcium nephrolithiasis (8 male, 13 female) | Drink 2 L of different types of mineral water of low, medium and high calcium content for one month. Note: Low-calcium water pH 7.1; Medium-calcium water pH 7.2; High-calcium water pH 6.1 |
21 | Baseline: measured during the pre-phase | 21 | 24h urinary uric acid, pH, urine volume Note: combining data from different types of mineral water groups during overall analyses |
| OV Konstantinova, et al. (2013) | Russia | Self-controlled study | 47 adult patients with urolithiasis and chronic pyelonephritis and 3 children; 14 patients dropped out | Drink mineral water "Naftusya" of Zbruchansk field at a dose 200 mL 3 times a day for adults, 50-150 mL 3 times a day for children, for 7-20 days | 36 | Baseline: measured during the pre-phase | 36 | 24h urinary uric acid, pH, urine volume |
| Shuangyang Peng, et al (2007) | China | Self-controlled study | 81 undergoing minimally invasive lithotomy, 2 dropped | Drink water 2-2.5 L daily without special diet prevention for 6 months | 79 | Baseline: measured during the pre-phase | 81 | 24h urinary uric acid |
| Rodgers AL, et al (1997) | South Africa | Self-controlled study | 20 healthy males; 20 health females; 20 male caltium oxalate kidney stone formers; 20 female calcium oxalate kidney stone formers |
Drink 1.5 L of Vittel mineral water during each of 3 consecutive days without any change to their normal diet; then drink 1.5 L of tap water during each of 3 consecutive days | 80 | Baseline: measured during normal dietary conditions | 80 | 24h urinary uric acid, pH, urine volume; relative supersaturation of uric acid Note: combining data from tap water and mineral water groups during overall analyses |
| R.Iantorno-M, et al. (1997) | Italy | Self-controlled study | Patients with calcium kidney stones (13 male, 7 female) | Drink a low mineral content water “Monteferrante” 2.5-3 L daily for 6 months | 20 | Baseline: measured during the pre-phase | 20 | 24h urinary uric acid, urine volume Note: combining data of urinary uric acid from follow-up 3 and 6 months during overall analyses |
| R Siener, et al (2004) | Germany | Self-controlled study | Healthy subjects | Drink 1.4 L of mineral water daily for 4 weeks with usual diet | 12 | Baseline: measured during the pre-phase with usual diet and beverages, monitored for two weeks | 12 | 24h urinary uric acid, pH, urine volume Note: combining data from two control weeks and four follow-up weeks respectively during overall analyses |
| Stacey G. Koff, et al. (2007) | America | Self-controlled study | Patients with kidney stones | Drink enough fluid to urinate at least 2 L daily. Fluid intake: 30 mL of lemon juice with three-fourths cup of water and sweetener for each serving, three times daily for 5 days, within no less than 2 weeks washout period | 21 | Baseline: measured during the pre-phase | 21 | 24h urinary uric acid, pH, urine volume |
| Torsten Keûler, et al. (2000) | Germany | Self-controlled study | Healthy male subjects with no previous history of urinary calculi or other renal disorders. | Drink a neutral fruit tea with no influence on the urinary with bicarbonate-rich mineral water 500 mL, four times daily for 2days |
24 | Baseline: measured during the pre-phase | 24 | 24h urinary uric acid, pH, urine volume; relative supersaturation of uric acid |
| Study excluded form quantitative synthesis | ||||||||
| Milewski JB, et al. (1987) | Poland | Self-controlled study | Patients with hyperuricemia and hyperuricuria, patients with uric acid stones | 24 patients with uric acid stones drank mineral water from the Dabrowka spring at the Szczawno-Zdroj, 30ml / kg body weight for three weeks. | 24 | Drink less amount of water daily | 26 | 24h urinary uric acid, pH Note: excluded this study in quantitative synthesis as standard deviation were missing from the original report |
| Neimark AI, et al. (2003) | Russia | Self-controlled study | Patients with nephrolithiasis were exposed to extracorporeal shock-wave lithotripsy | 10 patients drank tap water and 17 patients drank silver-containing mineral water Serebryany klyuch, 250-300 mL per service, 5-7 times daily for 7-8 days after extracorporeal shock-wave lithotripsy | 27 | Baseline: measured during the pre-phase | 27 | 24h urinary uric acid Note: excluded this study in quantitative synthesis as the unbelievable data were reported in the original study (after treatment 24 h urinary uric acid excretion changed from0.8±2.4 to 8.3±2.23 mmol/d) |
Definition of high water intake
Fifteen studies specifically defined high water intake. Two studies calculated daily water intake volumes based on the volunteers’ lean body weight [25,27]; one study calculated daily water intake volumes by 120 mL per 100 kcal/d [34]; one study described daily water intake by cups [30]. Sixteen studies reported the intervention as more than 1.2L of water intake, one study had unclear increased water intake [27], one study described high daily water intake as evidenced by a urine volume of at least 2L [12]. Only two studies described water or tea as an extra fluid intake beside daily water consumption, with the amount less than 1L [30,39], which needed add up other forms of water (beverage, fluid ingested from solid foods or other types of water intake) as total water intake daily. Three studies counted green tea [30], fruit tea [33], bag tea [40] drinking as the daily water consumption. Details were shown in table 1.
Overall analysis and sensitivity analysis
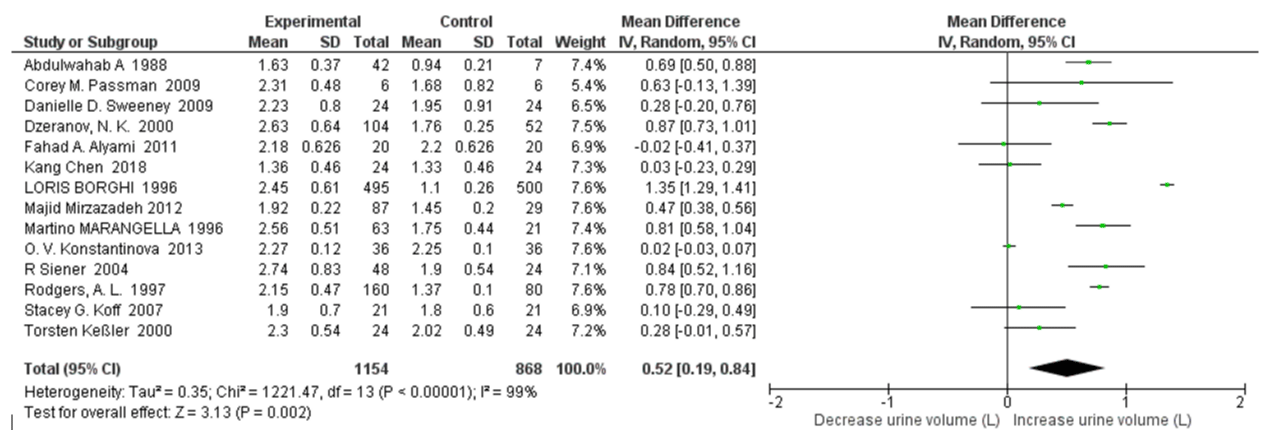
24h Urine Volume with High Water Intake: Fourteen studies were included in the quantitative synthesis of 24h urine volume with high water intake. In overall analyses, high water intake individuals had a significantly increased urine volume versus controls (SMD=0.52 L; 95% CI: 0.19, 0.84; p=0.002). High heterogeneity was apparent between all qualified studies, with an I2 =99% and Pheterogeneity <0.00001 (Figure 2). A sensitivity analysis was also performed by removing 1 study at a time to see whether the omission of the study influenced the overall results. None of included studies would be excluded, since there was no significant decrease the I2 when omission of each study at a time.
Figure 2: Forest plot of included studies comparing 24h urine volume in individuals with vs without high water intake; using random-effects meta-analysis. Diamond data markers, pooled SMD and 95% CIs for outcomes of interest.
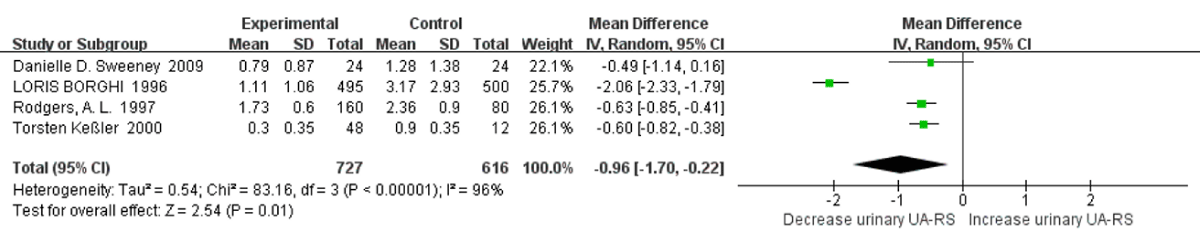
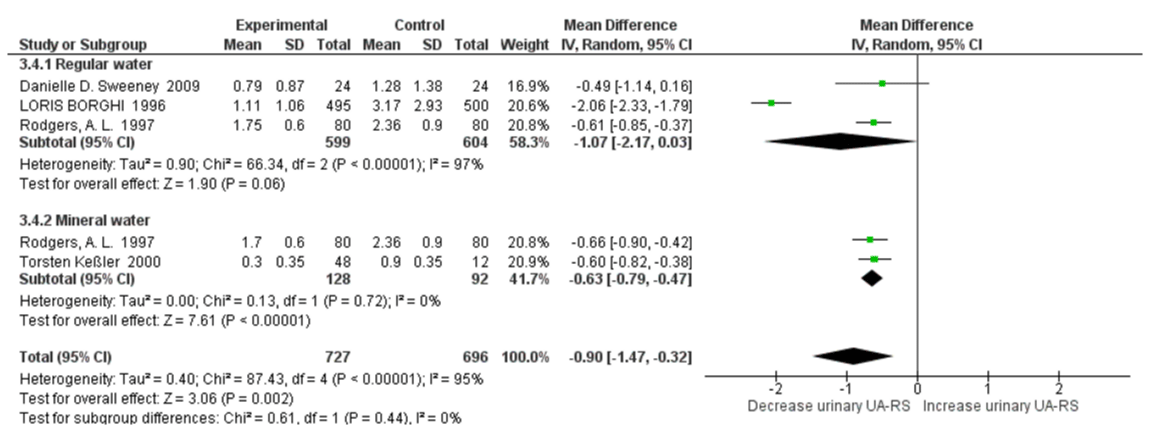
Relative Super saturation of Uric Acid with High Water Intake: Four studies were included in a quantitative synthesis of relative supersaturation of uric acid with high water intake. The risk factor of relative supersaturation of uric acid decreased significantly versus controls (SMD=-0.96; 95% CI: -1.70, -0.22; p=0.01). High heterogeneity was apparent between all qualified studies, with an I2=96% and Pheterogeneity <0.00001 (Figure 3). In a sensitivity analyses, the pooled SMD of relative supersaturation of uric acid changed to -0.61 (95% CI: -0.76, -0.46; p<0.00001) with a statistical significance remaining after omission of the LOURIS BORGHI Study. The heterogeneity was also reduced with this omission, with an I2=0% and Pheterogeneity =0.92.
Figure 3: Forest plot of included studies comparing relative supersaturation of uric acid in individuals with vs without high water intake; using random-effects meta-analysis. Diamond data markers, pooled SMD and 95% CIs for outcomes of interest.
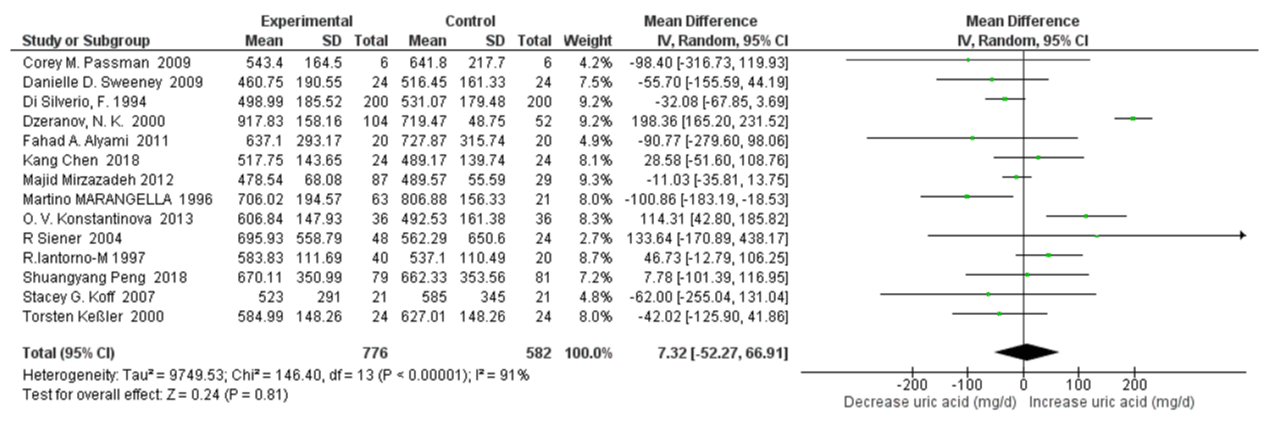
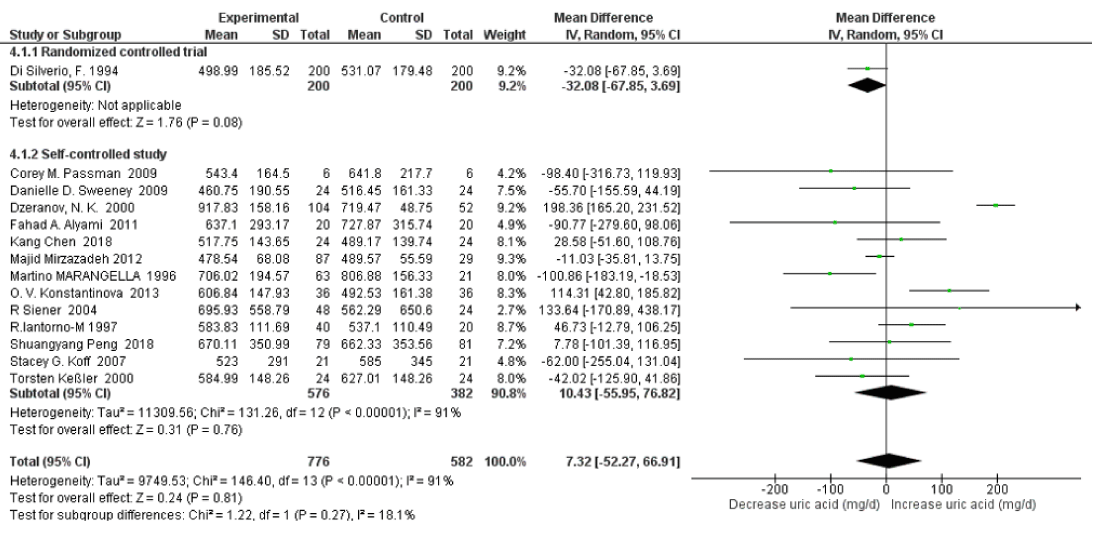
24 h Urinary Uric Acid Excretion with High Water Intake: Fourteen studies were included in quantitative synthesis of 24h urinary uric acid excretion with high water intake. The risk factor of urinary uric acid was not significantly related to high water intake (SMD=7.32mg/d, 95% CI: -52.27, 66.91; p=0.81). A high heterogeneity was apparent with an I2=91% and Pheterogeneity <0.00001 (Figure 4). In sensitivity analyses, the pooled SMD of 24h urinary uric acid decreased to -7.31mg/d (95% CI: –39.30, 24.67; p=0.65) when omitting the Dzeranov, N. K. Study and decreased to -17.98mg/d (95% CI: –41.65, 5.69; p=0.14) when omitting the O. V. Konstantinova Study further. The heterogeneity was also reduced, with I2=54% Pheterogeneity=0.01 and I2=21% Pheterogeneity=0.24 respectively.
Figure 4: Forest plot of included studies comparing 24h urinary uric acid excretion in individuals with vs without high water intake; using random-effects meta-analysis. Diamond data markers, pooled SMD and 95% CIs for outcomes of interest.
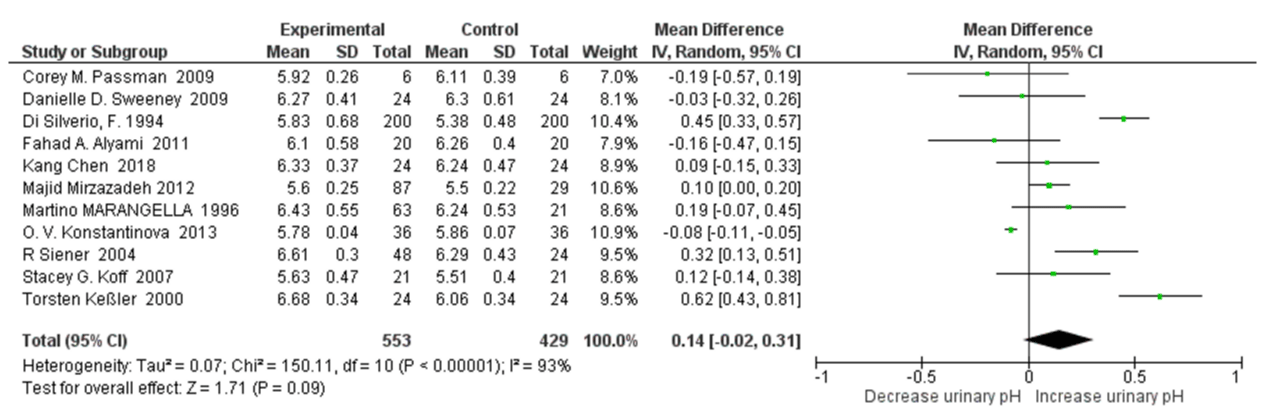
24 h Urinary pH with High Water Intake: Eleven studies were included in quantitative synthesis of 24h urinary pH with high water intake. The UAN risk factor urinary pH was not significantly related to high water intake (SMD=0.14, 95% CI: -0.02, 0.31; p=0.09).). It appeared a high heterogeneity, with an I2=93% and Pheterogeneity <0.00001 (Figure 5). In sensitivity analyses, as omitting the O. V. Konstantinova Study led the results to become statistically significance versus controls, with the pooled SMD of relative 24h urinary pH changed to 0.18 (95% CI: 0.02, 0.33; p=0.02) . The heterogeneity was also reduced, with an I2=83% and Pheterogeneity <0.00001.
Figure 5: Forest plot of included studies comparing 24h urinary pH in individuals with vs without high water intake; using random-effects meta-analysis. Diamond data markers, pooled SMD and 95% CIs for outcomes of interest.
Subgroup analysis and sensitivity analysis
Subgroup analyses were conducted regarding subject category (healthy subjects vs stone formers), the type of water intake (regular water vs mineral water), and study type (Randomized Controlled Trial vs Self-controlled Study).
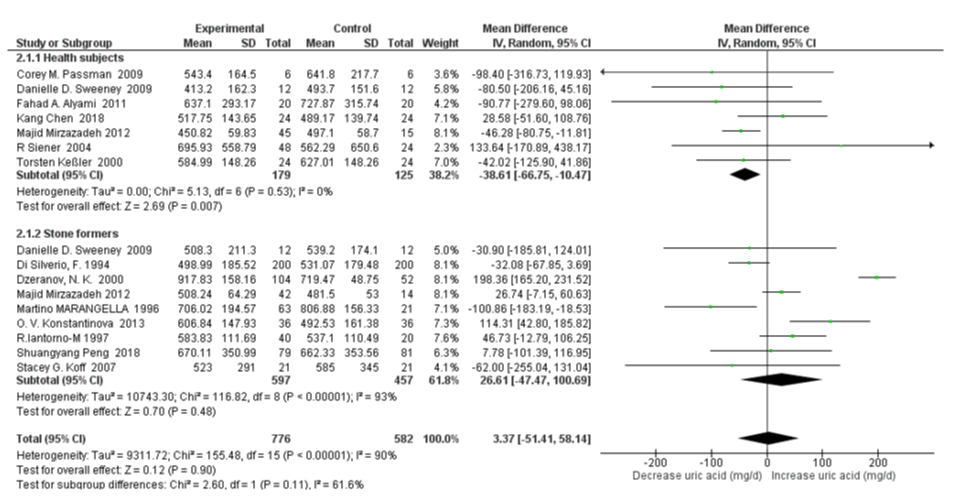
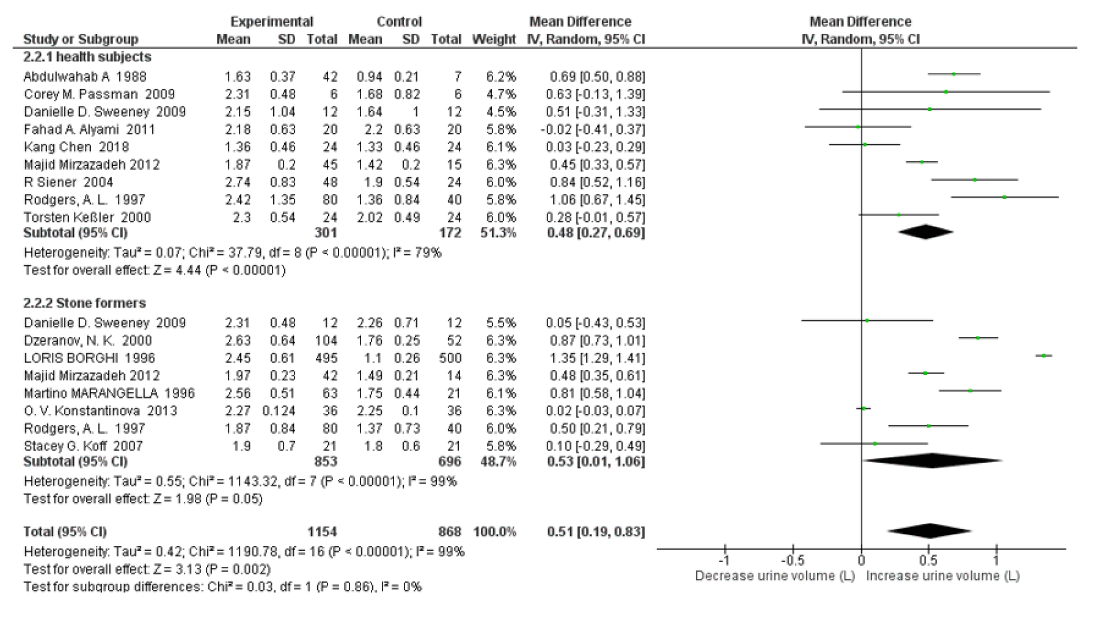
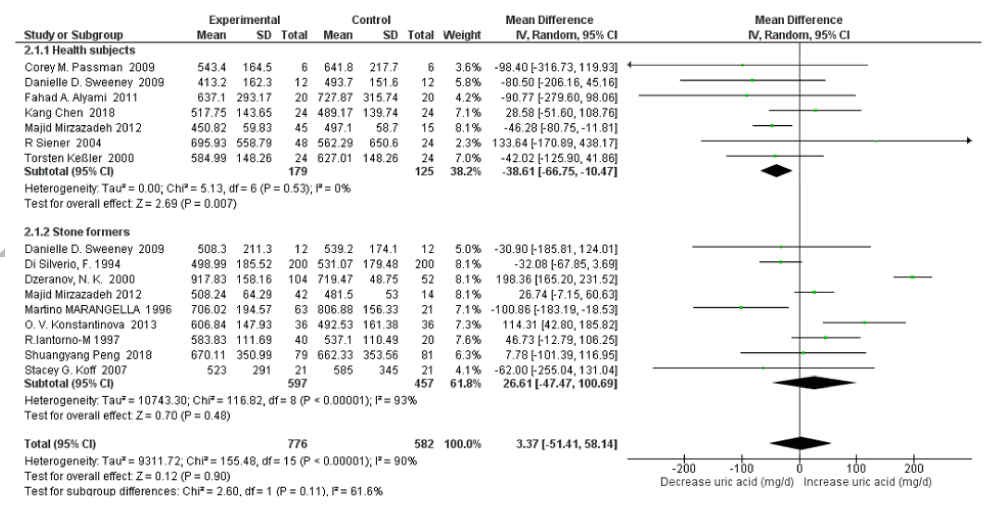
Subgroup Analysis on Healthy Subjects vs Stone Formers: For 24h urinary uric acid, subgroup analysis stone former status revealed that there was moderate heterogeneity between healthy subjects and stone formers with an I2=61.6%, Pheterogeneity=0.11; and high water intake was associated with significantly decreased only in healthy subjects (SMD=-38.61 mg/d, 95% CI: -66.75, -10.47; p=0.007; I2=0%, Pheterogeneity=0.53); with no significant changes in stone formers (SMD=26.61 mg/d, 95% CI: -47.47, 100.69; p=0.48; I2=93%, Pheterogeneity<0.00001) (Figure 6). When omitting the Dzeranov NK. In the stone former group, the pooled SMD of 24h urinary uric acid decreased to 5.92mg/d (95% CI: –38.68, 50.51; p=0.79; I2=70%, Pheterogeneity=0.001); when omitting the OV Konstantinova Study further the pooled SMD decreased to -9.37mg/d (95% CI:–49.26, 30.53; p=0.65; I2=58%, Pheterogeneity=0.03). The subgroup analysis revealed that there was no significant heterogeneity for 24h urine volume between healthy subjects and stone formers with an I2=0%, Pheterogeneity=0.86. High water intake was associated with significantly increased 24h urine volume SMD=0.48 L/d (95% CI: 0.27, 0.69; p<0.00001; I2=79%, Pheterogeneity<0.00001) both in healthy subjects and SMD=0.53 L/d (95% CI: 0.01, 1.06; p=0.05; I2=99%, Pheterogeneity<0.00001) in stone formers (Figure 7).
Figure 6: Forest plot of included studies comparing 24h urinary uric acid excretion in subgroup analyses on the subject condition; using random-effects meta-analysis. Diamond data markers, pooled SMD and 95% CIs for outcomes of interest.
Figure 7: Forest plot of included studies comparing 24h urine volume in subgroup analyses on the subject condition; using random-effects meta-analysis. Diamond data markers, pooled SMD and 95% CIs for outcomes of interest.
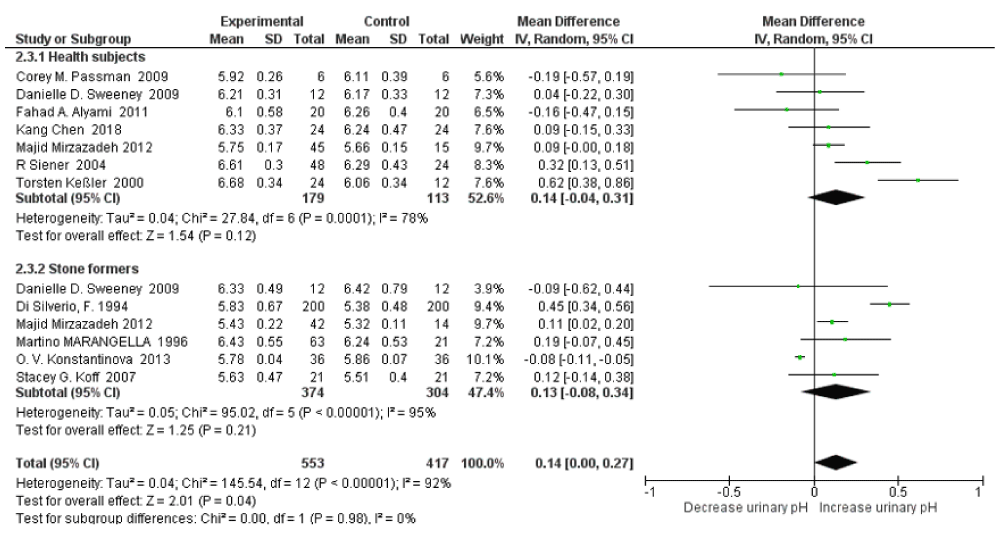
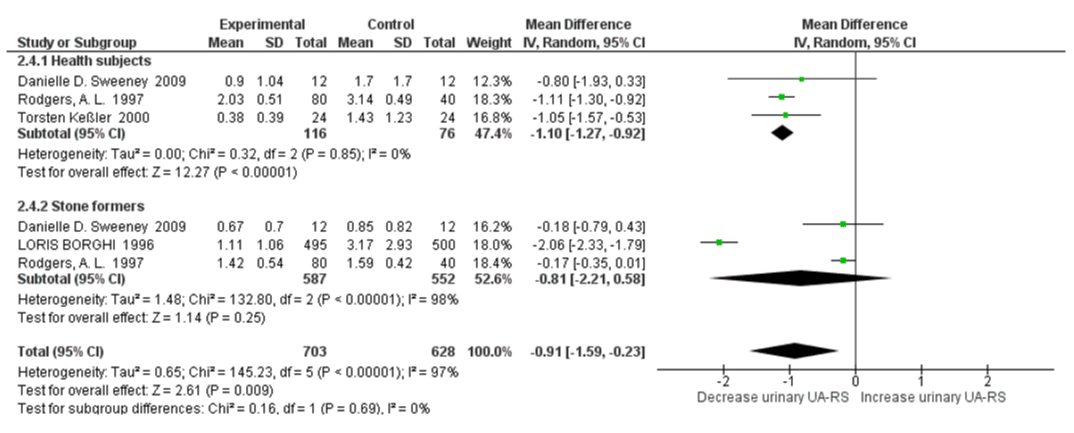
The subgroup analysis revealed that there was no significant heterogeneity between healthy subjects and stone formers for 24h urine pH, with an I2=0%, Pheterogeneity=0.98. There was slightly increased urinary pH with high water intake SMD=0.14 (95% CI: -0.04, 0.31; p=0.12; I2=78%, Pheterogeneity=0.0001) in healthy subjects and SMD=0.13 (95% CI: -0.08, 0.34; p=0.21; I2=95%, Pheterogeneity<0.00001) in stone formers (Figure 8). For relative supersaturation of uric acid, the subgroup analysis revealed that there was no significant heterogeneity between healthy subjects and stone formers with an I2=0%, Pheterogeneity=0.69. Significantly decreased relative supersaturation of uric acid was only observed with high water intake in healthy subjects, with SMD=-1.10 (95% CI: -1.27, -0.92; p<0.00001; I2=0%, Pheterogeneity=0.85); and slightly decreased in stone formers, with SMD=-0.81 (95% CI: -2.21, 0.58; p=0.25; I2=98%, Pheterogeneity<0.00001) (Figure 9). When omitting the LORIS BORGHI Study in stone former subgroup, the pooled SMD of relative supersaturation of uric acid decreased significantly -0.17 (95% CI: –0.34, -0.00; p=0.05; I2=0%, Pheterogeneity=0.98).
Figure 8: Forest plot of included studies comparing 24h urinary pH in subgroup analyses on the subject condition; using random-effects meta-analysis. Diamond data markers, pooled SMD and 95% CIs for outcomes of interest.
Figure 9: Forest plot of included studies comparing relative supersaturation in subgroup analyses on the subject condition; using random-effects meta-analysis. Diamond data markers, pooled SMD and 95% CIs for outcomes of interest.
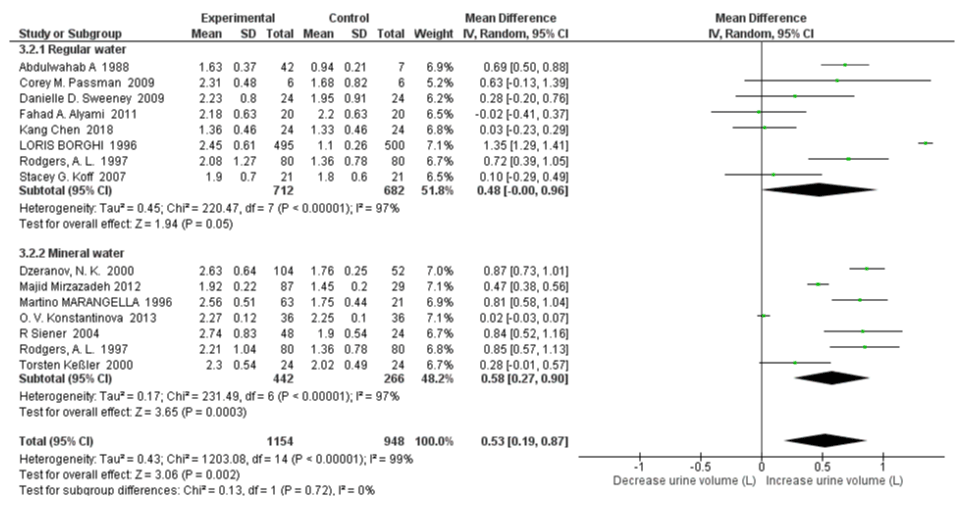
Subgroup Analysis on Regular Water vs Mineral Water: There was moderate heterogeneity between regular water and mineral water subgroups for 24 hr uric acid excretion with an I2=70.5%, Pheterogeneity=0.07. Uric acid excretion was significantly decreased only in regular water intake group (SMD=-61.49 mg/d, 95% CI: -120.74, -2.24; p=0.04; I2=31%, Pheterogeneity=0.20); with no significant changes in the mineral water studies (SMD=31.79 mg/d, 95% CI: -47.87, 111.44; p=0.43; I2=95%, Pheterogeneity<0.00001) (Figure 10). When omitting the Dzeranov NK. Study in mineral water group, the pooled SMD of 24h urinary uric acid decreased to -0.53 mg/d (95% CI: –43.67, 42.61; p=0.98; I2=73%, Pheterogeneity=0.0009); when omitting the O. V. Konstantinova Study further the pooled SMD decreased to -18.85 mg/d (95% CI:–52.63, 14.92; p=0.27; I2=53%, Pheterogeneity=0.06). There was no significant heterogeneity between the regular water and mineral water groups with an I2=0%, Pheterogeneity=0.72. High water intake was associated with significantly increased 24h urine volume both in the regular water group and mineral water group with SMD=0.48 L/d (95% CI: -0.00, 0.96; p=0.05; I2=97%, Pheterogeneity<0.00001) and SMD=0.58 L/d (95% CI: 0.27, 0.90; p=0.0003; I2=97%, Pheterogeneity<0.00001) respectively (Figure 11).
Figure 10: Forest plot of included studies comparing 24h urinary uric acid excretion in subgroup analyses on water type; using random-effects meta-analysis. Diamond data markers, pooled SMD and 95% CIs for outcomes of interest.
Figure 11: Forest plot of included studies comparing 24h urine volume in subgroup analyses on water type; using random-effects meta-analysis. Diamond data markers, pooled SMD and 95% CIs for outcomes of interest.
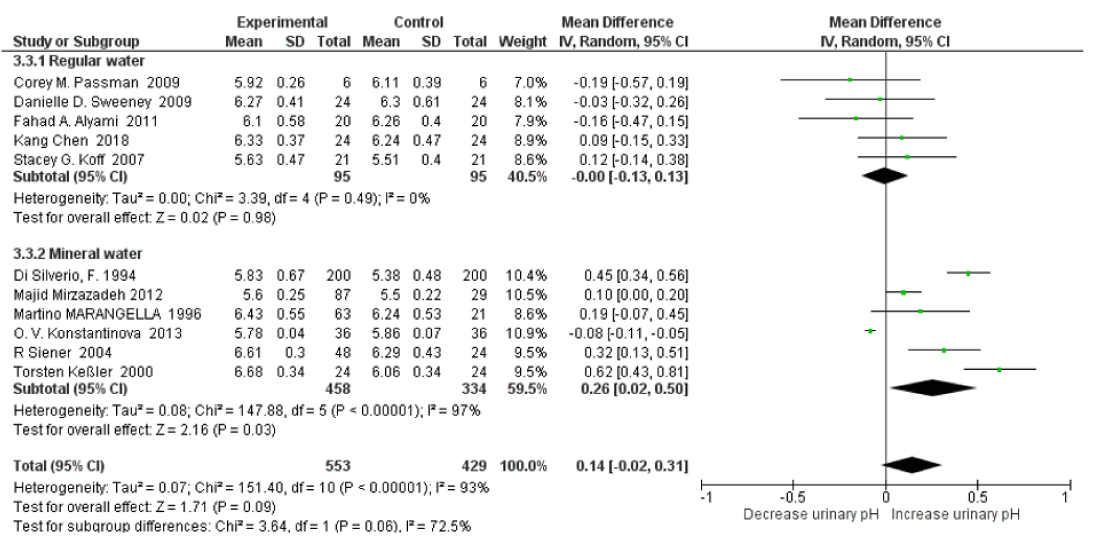
There was moderate heterogeneity between the regular water and mineral water groups for urinary pH with an I2=72.5%, Pheterogeneity=0.06. Urinary pH increased only in the mineral water group with SMD=0.26 (95% CI: 0.02, 0.31; p=0.03; I2=97%, Pheterogeneity<0.00001). There no significant changes of urinary pH in mineral water group with SMD=-0.00 (95% CI: -0.13, 0.13; p=0.98; I2=0%, Pheterogeneity<0.00001) (Figure 12). There was no significant heterogeneity between healthy subjects and stone formers with an I2=0%, Pheterogeneity=0.44. There was a significant decrease in relative supersaturation of uric acid only in the mineral water groups, with SMD=-0.63 (95% CI: -0.79, -0.47; p<0.00001; I2=0%, Pheterogeneity=0.72); and slightly decreased in regular water group, with SMD=-1.07 (95% CI: -2.17, 0.03; p=0.06; I2=97%, Pheterogeneity<0.00001) (Figure 13). When omitting the BORGHI study, the pooled SMD of relative supersaturation of uric acid decreased significantly with SMD=-0.60 (95% CI: –0.82, -0.37; p<0.00001; I2=0%, Pheterogeneity=0.73).
Figure 12: Forest plot of included studies comparing 24h urinary pH in subgroup analyses on water type; using random-effects meta-analysis. Diamond data markers, pooled SMD and 95% CIs for outcomes of interest.
Figure 13: Forest plot of included studies comparing relative supersaturation of uric acid in subgroup analyses on water type; using random-effects meta-analysis. Diamond data markers, pooled SMD and 95% CIs for outcomes of interest.
Subgroup Analysis on Randomized Controlled Trial vs Self-controlled Study: Subgroup analysis via study type revealed that there was high heterogeneity between randomized controlled trial and self-controlled studies for 24h uric acid excretion with an I2=91%, Pheterogeneity<0.00001. There were no significant changes in uric acid excretion in both randomized controlled trials (SMD=-32.08 mg/d, 95% CI: -67.85, 3.69; p=0.08; Not applicable heterogeneity) and self-controlled studies (SMD=10.43 mg/d, 95% CI:-55.95, 76.82; p=0.76; I2=91%, Pheterogeneity<0.00001) respectively (Figure 14). After omitting the Dzeranov study the pooled SMD of 24h urinary uric acid decreased to -3.79 mg/d (95% CI: –41.57, 33.99; p=0.84; I2=54%, Pheterogeneity=0.01); Omitting the Konstantinova study the pooled SMD further decreased to -14.90 mg/d (95% CI:–44.18, 14.38; p=0.32; I2=23%, Pheterogeneity=0.23), and after omitting the Iantorno-M study the pooled SMD decreased to -18.82 mg/d (95% CI:–39.47, 1.83; p=0.07; I2=0%, Pheterogeneity=0.46) .
Figure 14: Forest plot of included studies comparing 24h urinary uric acid excretion in subgroup analyses on study type; using random-effects meta-analysis. Diamond data markers, pooled SMD and 95% CIs for outcomes of interest.
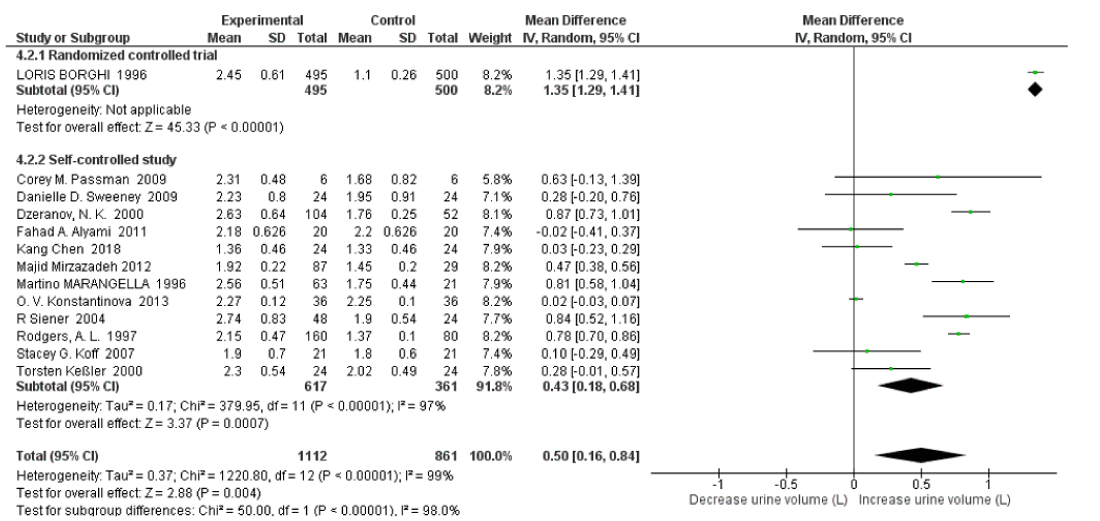
There was high heterogeneity between randomized controlled trials and self-controlled studies for 24hr volume with an I2=98%, Pheterogeneity<0.00001. High water intake was associated with significantly increased 24h urine volume both in randomized controlled trials and self-controlled studies with SMD=1.35 L/d (95% CI: -1.29, 1.41; p<0.00001; Not applicable heterogeneity) and SMD=0.58 L/d (95% CI: 0.27, 0.90; p=0.0003; I2=97%, Pheterogeneity<0.00001) respectively (Figure 15).
Figure 15: Forest plot of included studies comparing 24h urine volume in subgroup analyses on study type; using random-effects meta-analysis. Diamond data markers, pooled SMD and 95% CIs for outcomes of interest.
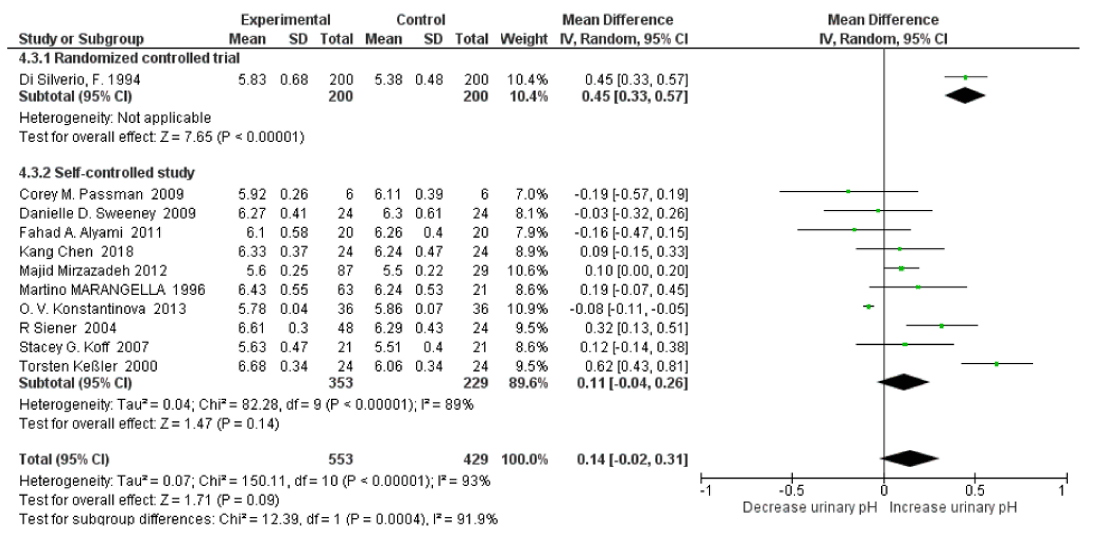
The subgroup analysis revealed that there was high heterogeneity between randomized controlled trials and self-controlled studies for urine pH with an I2=91.9%, Pheterogeneity=0.00004. T Urine pH significantly increased only in the randomized controlled trials with SMD=0.45 (95% CI: 0.33, 0.57; p=0.03; Not applicable heterogeneity). There no significant changes in the mineral water group with SMD=0.11 (95% CI: -0.04, 0.26; p=0.14; I2=89%, Pheterogeneity<0.00001) (Figure 16). After omitting the Konstantinova study the pooled SMD of 24h urinary pH increased to 0.14 (95% CI: –0.01, 0.30; p=0.07; I2=78%, Pheterogeneity<0.00001); after omitting the Torsten Keûler study the pooled SMD changed to 0.09 (95% CI:–0.01, 0.02; p=0.07; I2=38%, Pheterogeneity<0.0001); and after omitting the Siener Study further the pooled SMD changed to -0.07 (95% CI:–0.00, 0.15; p=0.05; I2=0%, Pheterogeneity=0.45). The subgroup analysis revealed that there was high heterogeneity between randomized controlled trials and self-controlled studies for relative SS with an I2=98.8%, Pheterogeneity<0.00001. Relative supersaturation of uric acid fell both in randomized controlled trials and self-controlled studies with an SMD=-2.06 (95% CI: -2.33, -1.79; p<0.00001; Not applicable heterogeneity) and SMD=-0.61 (95% CI: -0.76, -0.46; p<0.00001; I2=0%, Pheterogeneity<0.90) (Figure 17). After omitting the Torsten Keûler Study the pooled SMD of relative supersaturation of uric acid decreased with a SMD=-0.62 (95% CI: –0.83, -0.42; p<0.00001; I2=0%, Pheterogeneity=0.67).
Figure 16: Forest plot of included studies comparing 24h urinary pH subgroup analyses on study type; using random-effects meta-analysis. Diamond data markers, pooled SMD and 95% CIs for outcomes of interest.
Figure 17: Forest plot of included studies comparing relative supersaturation of uric acid subgroup analyses on study type; using random-effects meta-analysis. Diamond data markers, pooled SMD and 95% CIs for outcomes of interest.
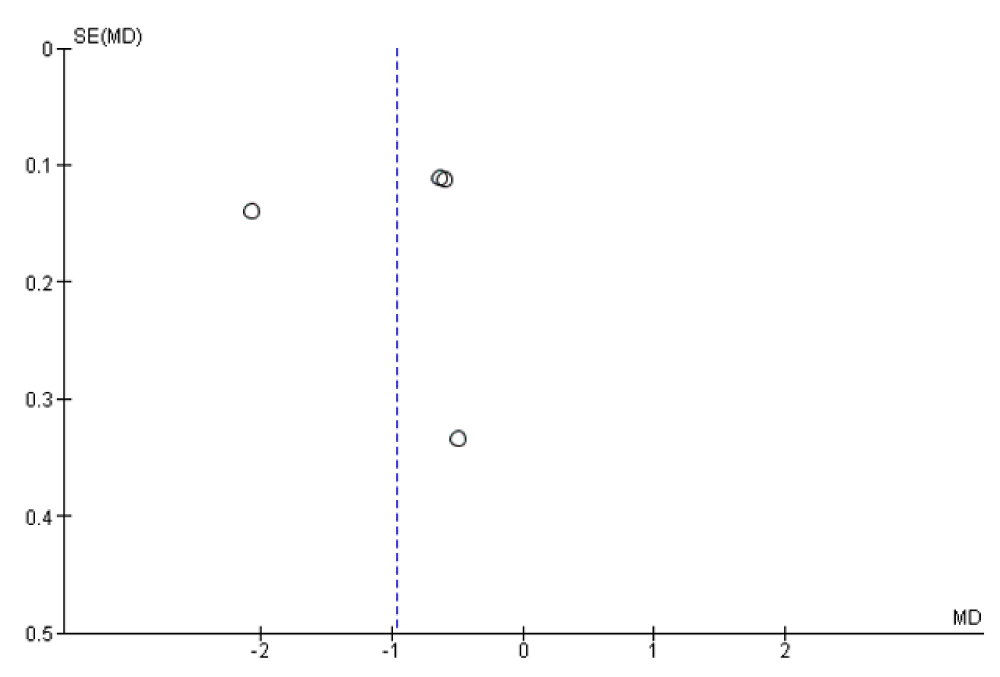
Evaluation for publication bias
Assessment of publication bias was completed with a funnel summarized in figures 18-21. The overall funnel plot was moderately asymmetrical in shape confirming a publication bias.
Figure 18: Funnel plot of included studies in the meta-analysis for the risk factor of 24h urinary uric acid excretion with and without high water intake. MD=Mean difference, SE = standard error.
Figure 19: Funnel plot of included studies in the meta-analysis for the risk factor of 24h urine volume with and without high water intake. MD=Mean difference, SE = standard error.
Figure 20: Funnel plot of included studies in the meta-analysis for the risk factor of 24h urinary pH with and without high water intake. MD=Mean difference, SE = standard error.
Figure 21: Funnel plot of included studies in the meta-analysis for the risk factor of relative supersaturation of uric acid with and without high water intake. MD=Mean difference, SE = standard error.
Strength and limitations
The present study’s comprehensive literature search with no languages restrictions and initial date of publication lends credibility to the results. The studies were conducted in eight countries, including developed and developing countries. To our knowledge, this is the first study employing a meta-analysis that evaluated the association of high water intake for preventing the risk of uric acid kidney stones, providing a more refined analysis than some prior studies. The strengths of our study were that it assessed the magnitude of the effects of high water intake for preventing uric acid nephrolithiasis, and synthesized the standard mean difference changes of individual urinary profiles. The results suggested that high water intake likely had effect to prevent uric acid nephrolithiasis. Furthermore, subgroup and sensitivity analyses suggested that our study’s results were robust in most cases, which strengthens the conclusions of our study. Certain limitations of this study should be considered. The first is the variability of water intervention. The included studies used several types of water including mineral water (with low hardness 110 mg/L, moderate hardness 180 mg/L, and high hardness 280 mg/L), regular water (tap water, carbonated, boiling water, bottled water, distilled water, tea, et al.). Only three studies described the pH of consumed water [30,34,36]. The volume and duration of water intake also varied. Furthermore we get water not only directly as water intake but from solid forms of food, fruits and vegetables, and also a small amount of metabolic water [43-45]. Although the water intake varied, the comparison was between water intervention and baselines or controls, and in most studies the diet was the subject’s usual diet. There was high heterogeneity in the overall analyses for 24h urinary uric acid excretion, urine volume, urinary pH and relative supersaturation of uric acid. However, in a sensitivity analysis for 24h urine volume and relative supersaturation of uric acid, when 1 or 2 studies are omitted to reduce heterogeneity, the results remained significant. In a sensitivity analysis for 24h urinary pH when the Konstantinova study was omitted the results became statistically significant.
Publication bias is one of the most important issues in meta-analysis, and we cannot deny the possibility of such bias [46]. Although the literature search was performed with no languages restrictions and beginning date of publication, we could have inadvertently missed eligible studies. There could be publication bias in this present systematic review. Indeed the funnel-plot assessment showed evidence for publication bias in this meta-analysis [47]. In present study the sensitive analysis indicated that the analysis used a small number of studies with high heterogeneity, which could also introduce bias. However, considering that the quality of studies of water intake may be affected by many confounding biases, these limitations may be acceptable. The subgroup analyses were similar to those of the overall analyses. The third limitation was the study design and small number of participants. This systematic review included 895 participants, and the number of participants per study ranged from 6 to 100. Most of the studies were self-controlled; only two RCT studies with 399 participants were included. Research indicates that random errors induced by a small numbers of studies might cause bias in the results of meta-analyses [48]. Furthermore, it was difficult to include placebo control in water intake intervention, so that self-controlled studies were performed in most. However, the subgroup analysis of study type indicated that similar changes of urine volume, relative supersaturation of uric acid and 24h urinary pH were observed in randomized controlled trials and self-controlled studies.
Discussion
The prevalence of UAN is increasing recent years, and now accounts for approximately 7% to 10% of kidney stone formers [1,3]. Multiple factors contribute to the formation of uric acid kidney stones. The most important risk factor is persistently low urinary pH, followed by low urinary volume, and hyperuricosuria [7,49,50]. When urinary pH falls below 5.5, urinary urate exists largely as uric acid which can precipitate as uric acid crystals [51,52]. Hyperuricosuria is the second risk factor of UAN [50]. Low urinary output also increases the concentration of lithogenic solutes [53]. The prevention and treatment of UAN thus includes urine alkalinization as well as hydration (increasing urine volume above 2000 ml daily) [54,55]. Medical dissolution treatment including urine dilution, fluid intake, and alkalization is effective in many [8]. This is the first meta-analysis to confirm a significant association between high water intake and a lower risk of uric acid kidney stones. The overall analysis indicated that high water intake increased 24h urine volume (SMD=0.52 L; 95% CI: 0.19, 0.84; p=0.002) and decreasing relative supersaturation of uric acid (SMD=-0.96; 95% CI: -1.70, -0.22; p=0.01). Sensitivity analyses showed unstable results for 24h urinary pH. However after omitting the Konstantinova study results became statistically significant (SMD=0.18; 95% CI: 0.02, 0.33; p=0.02) After omitting the Dzeranov and Konstantinova studies results for UA excretion reversed with the SMD changing from 7.32mg/d (95% CI: -52.27, 66.91; p=0.81) to -17.98mg /d (95% CI: –41.65, 5.69; p=0.14). Further subgroup analyses revealed similar results for 24h urine volume and relative supersaturation of uric acid. The results persisted in a number of subgroup and sensitivity analyses, suggesting that the findings are not likely to be completely explained by confounding. In subgroup analysis 24h urinary pH significantly increased (Total SMD=0.14; 95% CI: 0.00, 0.27; p=0.04) with similar increases in healthy subjects (Subtotal SMD=0.14; 95% CI: -0.04, 0.31; p=0.12) and stone formers (Subtotal SMD=0.13; 95% CI: -0.08, 0.34; p=0.21). Subgroup analyses revealed high water intake decreased uric acid excretion more in healthy subjects than stone formers, with SMD -38.61 mg/d vs 26.61 mg/d; 24h urinary pH subgroup analyses shown significantly increased pH in mineral water intake group vs regular water intake group, with SMD 0.14 vs -0.00. This result suggests high water intake can significantly reduce relative supersaturation of uric acid and increase urine volume. Even though weak, the observed reduction in 24h urinary uric acid excretion and increased urinary pH with high water intake should favor uric acid crystal dissolution in renal tubules and flushing away of uric acid micro-crystals [12].
Overall, this meta-analysis presents evidence that high water intake intervention can reduce the risk factors of uric acid kidney stones. Drinking water is a simple and cost-effective preventive method for kidney stone prevention, and is recommended by most healthcare facilities and clinical guidelines [14,15,56,57]. The current findings heave important public health implications in light of the current epidemic of uric acid kidney stones [3,58]. Recent epidemiological studies suggest that kidney stones are a risk factor for acute and chronic kidney injury [59,60], kidney cancer [61], metabolic disorders [62], and cardiovascular disease [63]. Therefore water intake for prevention of uric acid stones has multiple potential benefits.
Conclusion
High water intake can help prevent UAN by increasing urine volume, decreasing UA concentration, and depending on the fluid type increasing urinary pH, thus decreasing relative super saturation of uric acid. Current evidence is not strong due to a relatively small number of studies and participants. Thus high quality clinical studies should be completed to confirm the effects of water intake for prevention of UAN.
References
- Wiederkehr MR, Moe OW. Uric Acid Nephrolithiasis: A Systemic Metabolic Disorder. Clin Rev Bone Miner Metab. 2011; 9: 207-217. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/25045326
- Ma Q, Fang L, Su R, Ma L, Xie G, et al. Uric acid stones, clinical manifestations and therapeutic considerations. Postgraduate Medical Journal. 2018; 94: 458-462. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/30002092
- Abou-Elela A. Epidemiology, pathophysiology, and management of uric acid urolithiasis: A narrative review. Journal of Advanced Research. 2017; 8: 513-527. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/28748117
- Moe OW. Kidney stones: pathophysiology and medical management. The Lancet. 2006; 367: 333-344. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/16443041
- Sakhaee K. Epidemiology and clinical pathophysiology of uric acid kidney stones. Journal of Nephrology. 2014; 27: 241-245. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/24497296
- Portis AJ, et al. High Prevalence of Gouty Arthritis among the Hmong Population in Minnesota. Arthritis Care & Research. 2010; 62: 1386-1391. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/20506247
- Moe OW. Uric acid nephrolithiasis: proton titration of an essential molecule? Current Opinion in Nephrology and Hypertension, 2006; 15: 366-373. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/16775450
- Xu C, Zhang C, Wang XL, Liu TZ, Zeng XT, et al. Self-Fluid Management in Prevention of Kidney Stones: A PRISMA-Compliant Systematic Review and Dose-Response Meta-Analysis of Observational Studies. Medicine. 2015; 94: 1042. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/26166074
- Qaseem A, Dallas P, Forciea MA, Starkey M, Denberg TD, et al. Dietary and Pharmacologic Management to Prevent Recurrent Nephrolithiasis in Adults: A Clinical Practice Guideline from the American College of Physicians. Ann Intern Med. 2014; 161: 659-685. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/25364887
- Wang CJ, Grantham JJ, Wetmore JB. The medicinal use of water in renal disease. Kidney Int. 2013; 84: 45-53. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/23423255
- Bao YG, Wei Q. Water for preventing urinary stones. Cochrane Database of Systematic Reviews. 2012; 6.
- Borghi L, Meschi T, Amato F, Briganti A, Novarini A, et al. Urinary volume, water and recurrences in idiopathic calcium nephrolithiasis: a 5-year randomized prospective study. J Urol. 1996; 155: 839-843. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/8583588
- Fink HA, Akornor JW, Garimella PS, MacDonald R, Cutting A, et al. Diet, fluid, or supplements for secondary prevention of nephrolithiasis: a systematic review and meta-analysis of randomized trials. Eur Urol. 2009; 56: 72-80. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/19321253
- Pearle MS, et al. Medical management of kidney stones: AUA guideline. J Urol. 2014; 192: 316-324. PubMed:
- Fink HA, Goldfarb DS, Assimos DG, Curhan G, Denu-Ciocca CJ, et al. Medical management to prevent recurrent nephrolithiasis in adults: a systematic review for an American College of Physicians Clinical Guideline. Ann Intern Med. 2013; 158: 535-543. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/24857648
- Curhan GC, Willett WC, Speizer FE, Spiegelman D, Stampfer MJ. Comparison of dietary calcium with supplemental calcium and other nutrients as factors affecting the risk for kidney stones in women. Annals of Internal Medicine. 1997; 126: 497. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/9092314
- Taylor EN, Stampfer MJ, Curhan GC. Dietary factors and the risk of incident kidney stones in men: New insights after 14 years of follow-up. J Am Soc Nephrol. 2004; 15: 3225-3232. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/15579526
- Cheungpasitporn W, Rossetti S, Friend K, Erickson SB, Lieske JC. Treatment effect, adherence, and safety of high fluid intake for the prevention of incident and recurrent kidney stones: a systematic review and meta-analysis. Journal of Nephrology. 2016; 29: 211-219. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/26022722
- Linder BJ, Rangel LJ, Krambeck AE. The effect of work location on urolithiasis in health care professionals. Urolithiasis. 2013; 41: 327-331. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/23764693
- Sarica K, Inal Y, Erturhan S, Yağci F. The effect of calcium channel blockers on stone regrowth and recurrence after shock wave lithotripsy. Urological Research. 2006; 34: 184-189. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/16463053
- Koletsi D, Fleming PS, Michelaki I, Pandis N. Heterogeneity in Cochrane and non-Cochrane meta-analyses in orthodontics. J Dent. 2018; 74: 90-94. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/29738788
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003; 327: 557-560. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/12958120
- Da Costa BR, Nüesch E, Rutjes AW, Johnston BC, Reichenbach S, et al. Combining follow-up and change data is valid in meta-analyses of continuous outcomes: a meta-epidemiological study. J Clin Epidemiol. 2013; 66: 847-855. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/23747228
- Thabane L, Mbuagbaw L, Zhang S, Samaan Z, Marcucci M, et al. A tutorial on sensitivity analyses in clinical trials: the what, why, when and how. BMC Med Res Methodol. 2013; 13: 92-92. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/23855337
- Milewski JB, Krzeski T, Willak J. Effect of treatment with mineral water from the Dabrowka spring at the Szczawno-Zdroj health resort on uric acid levels of the blood and urine. Wiad Lek. 1987; 40: 875-878. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/3424837
- Neimark AI, Davydov AV. Use of "Serebrianyi Kliuch" mineral water in the postoperative treatment of patients with nephrolithiasis after extracorporeal shock-wave lithotripsy. Urologiia. 2003; 4: 44-46. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/12942727
- Passman CM, Holmes RP, Knight J, Easter L, Pais V, et al. Effect of soda consumption on urinary stone risk parameters. J Endourol. 2009; 23: 347-350. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/19275488
- Sweeney DD, Tomaszewski JJ, Ricchiuti DD, Averch TD. Effect of carbohydrate-electrolyte sports beverages on urinary stone risk factors. J Urol. 2009; 182: 992-997. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/19616798
- Koff SG, Paquette EL, Cullen J, Gancarczyk KK, Tucciarone PR et al. Comparison between lemonade and potassium citrate and impact on urine pH and 24-hour urine parameters in patients with kidney stone formation. Urology. 2007; 69: 1013-1016. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/17572176
- Chen K, Chen D, Lan C, Liang X, Zeng T, et al. Does green tea consumption increase urinary oxalate excretion? Results of a prospective trial in healthy men. Int Urol Nephrol. 2018; 50: 29-33. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/29052087
- Peng S. Clinical study of metabolic assessment in patients with renal calculi in diet prevention and treatment. International Medicine and Health Guidance News. 2018; 24: 2038-2042.
- Siener R, Jahnen A, Hesse A. Influence of a mineral water rich in calcium, magnesium and bicarbonate on urine composition and the risk of calcium oxalate crystallization. Eur J Clin Nutr. 2004; 58: 270-276. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/14749747
- Kessler T, Hesse A. Cross-over study of the influence of bicarbonate-rich mineral water on urinary composition in comparison with sodium potassium citrate in healthy male subjects. Br J Nutr. 2000; 84: 865-871. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/11177203
- Mirzazadeh M, Nouran MG, Richards KA, Zare M, et al. Effects of drinking water quality on urinary parameters in men with and without urinary tract stones. Urology. 2012; 79: 501-507. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/22173182
- Di Silverio F, D'Angelo AR. Prevention of renal calculosis: efficacy of Fiuggi water cure. Research Group on Renal Calculosis. Arch Ital Urol Androl. 1994; 66: 253-258. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/7812305
- Marangella M, Vitale C, Petrarulo M, Rovera L, Dutto F. Effects of mineral composition of drinking water on risk for stone formation and bone metabolism in idiopathic calcium nephrolithiasis. Clin Sci. 1996; 91: 313-318. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/8869414
- Iantorno R, Nicolai M, Ballone E, Passamonti M, Tenaglia R. Clinico-experimental study of low-mineral "Monteferrante" water: monitoring the recurrences in patients with calculi. Arch Ital Urol Androl. 1997; 69: 35-39. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/9181904
- Dzeranov NK, Beshliev DA, Golovanov SA, Kon'kova TA. Effects of mineral water TIB-2 on metabolic processes in urolithiasis patients. Urologiia. 2000; 3: 15-17. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/11186701
- Konstantinova OV, Dutov VV, Katibov MI, Trapeznikova MF, Ianenko ÈK. Experience in the use of mineral water "Naftusya" of Zbruchansk field in the treatment of patients with uroliths. Urologiia. 2013; 6: 9-13. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/24649756
- Noorwali AA. Tea Consumption as a Possible Risk Factor in Urolithiasis - a Preliminary-Report. Annals of Saudi Medicine. 1988; 8: 108-112.
- Alyami FA, Rabah DM. Effect of drinking parsley leaf tea on urinary composition and urinary stones' risk factors. Saudi J Kidney Dis Transpl. 2011; 22: 511-514. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/21566309
- Rodgers AL. Effect of mineral water containing calcium and magnesium on calcium oxalate urolithiasis risk factors. Urol Int. 1997; 58: 93-99. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/9096270
- Popkin BM, D'Anci KE, Rosenberg IH. Water, hydration, and health. Nutrition reviews. 2010; 68: 439-458.
- Guelinckx I, Tavoularis G, König J, Morin C, Gharbi H, et al. Contribution of Water from Food and Fluids to Total Water Intake: Analysis of a French and UK Population Surveys. Nutrients. 2016; 8: 630. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/27754402
- Nissensohn M, Sánchez-Villegas A, Galan P, Turrini A, Arnault N, et al. Beverage Consumption Habits among the European Population: Association with Total Water and Energy Intakes. Nutrients. 2017; 9. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/28406441
- Lunny C, Brennan SE, McDonald S, McKenzie JE. Toward a comprehensive evidence map of overview of systematic review methods: paper 2-risk of bias assessment; synthesis, presentation and summary of the findings; and assessment of the certainty of the evidence. Systematic Reviews. 2018; 7: 159. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/30314530
- Copas JB, Shi JQ. A sensitivity analysis for publication bias in systematic reviews. Stat Methods Med Res. 2001; 10: 251-265. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/11491412
- Tipton E. Small sample adjustments for robust variance estimation with meta-regression. Psychol Methods. 2015; 20: 375-393. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/24773356
- Pak CY, Sakhaee K, Peterson RD, Poindexter JR, Frawley WH. Biochemical profile of idiopathic uric acid nephrolithiasis. Kidney Int. 2001; 60: 757-761. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/11473659
- Pak CYC, Poindexter JR, Peterson RD, Koska J, Sakhaee K. Biochemical distinction between hyperuricosuric calcium urolithiasis and gouty diathesis. Urology. 2002; 60: 789-794. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/12429297
- Martillo MA, Nazzal L, Crittenden DB. The crystallization of monosodium urate. Current rheumatology reports. 2014; 16: 400-400.
- Siener R. Can the manipulation of urinary pH by beverages assist with the prevention of stone recurrence? Urolithiasis. 2016; 44: 51-56. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/26614113
- Kim S, Chang Y, Yun KE1, Jung HS, Lee SJ, et al. Development of Nephrolithiasis in Asymptomatic Hyperuricemia: A Cohort Study. Am J Kidney Dis. 2017; 70: 173-181. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/28410765
- Ferrari P, Bonny O. Diagnosis and prevention of uric acid stones. Ther Umsch. 2004; 61: 571-574. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/15493118
- Ngo TC, Assimos DG. Uric Acid nephrolithiasis: recent progress and future directions. Reviews in Urology. 2007; 9: 17-27. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/17396168
- Trinchieri A. Diet and renal stone formation. Minerva Med. 2013; 104: 41-54.
- Meschi T, Nouvenne A, Borghi L. Lifestyle recommendations to reduce the risk of kidney stones. Urol Clin North Am. 2011; 38: 313-320. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/21798393
- Prasad Sah OS, Qing YX, Associations between Hyperuricemia and Chronic Kidney Disease: A Review. Nephro-Urology Monthly. 2015; 7: e27233-e27233. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/26290849
- Tang X, Lieske JC. Acute and chronic kidney injury in nephrolithiasis. Current opinion in nephrology and hypertension. 2014; 23: 385-390. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/24848936
- Keddis MT, Rule AD. Nephrolithiasis and loss of kidney function. Current opinion in nephrology and hypertension. 2013; 22: 390-396.
- Cheungpasitporn W, Thongprayoon C, O'Corragain OA, Edmonds PJ, Ungprasert P, et al. The risk of kidney cancer in patients with kidney stones: a systematic review and meta-analysis. Qjm. 2015; 108: 205-212. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/25208892
- Hess B. Metabolic syndrome, obesity and kidney stones. Arab journal of urology. 2012; 10: 258-264. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/26558034
- Peng JP, Zheng H, Kidney stones may increase the risk of coronary heart disease and stroke: A PRISMA-Compliant meta-analysis. Medicine. 2017; 96: e7898-e7898. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/28834909