More Information
Submitted: March 23, 2022 | Approved: April 21, 2022 | Published: April 22, 2022
How to cite this article: Stea ED, Pronzo V, Pesce F, Fiorentino M, Mitrotti A, et al. Convalescent plasma therapy in aHUS patient with SARS-CoV-2 infection. J Clini Nephrol. 2022; 6: 036-039.
DOI: 10.29328/journal.jcn.1001088
Copyright License: © 2022 Stea ED, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: aHUS; Complement; Sars-CoV-2; Plasma
Convalescent plasma therapy in aHUS patient with SARS-CoV-2 infection
Emma Diletta Stea*, Virginia Pronzo, Francesco Pesce, Marco Fiorentino, Adele Mitrotti, Vincenzo Di Leo, Cosma Cortese, Annalisa Casanova, Sebastiano Nestola, Flavia Capaccio and Loreto Gesualdo
Department of Emergency and Organ Transplantation, Nephrology, Dialysis and Transplantation Unit, University of Bari, Bari, Italy
*Address for Correspondence: Stea Emma Diletta, A.O.U.C. Policlinic of Bari, P.zza G. Cesare, 11 70124 Bari (BA), Italy, Email: emmadiletta.stea@libero.it
Endotheliosis, thrombotic microangiopathy and complement system over activation have been described as pathologic features of tissue damage in the setting of coronavirus disease. Interestingly, complement-mediated cell injury is also a typical feature of atypical Hemolytic Uremic Syndrome. Indeed, a growing body of literature has described a higher risk of microangiopathy recurrence, in aHUS patients who test positive for SARS-CoV-2. The correct clinical and therapeutic management patients with a history of HUS and SARS-CoV-2 infection is not well established.
We report a case of SARS-CoV-2 infection in an aHUS patient who did not develop a recurrence of the disease and that was successfully treated with convalescent immune plasma therapy.
Endotheliosis has been described as a pathologic feature of organ damage in the setting of coronavirus disease 2019 (SARS-CoV-2) [1]. The involvement of complement system in the endothelial cell damage and in the pathogenesis of SARS-CoV-2 disease [1] has also been highlighted. Complement mediated endothelial-cell activation and injury, is a hallmark of atypical Hemolytic Uremic Syndrome (aHUS) [2]. On this premise, patients with aHUS history who test positive for SARS-CoV-2 have a higher risk of microangiopathy recurrence. We report the second case of SARS-CoV-2 infection in a patient with a history of aHUS who did not develop microangiopathy and was successfully treated with convalescent immune plasma therapy.
Statement of ethics
Written informed consent was obtained from the patient for publication of this case report and any accompanying images. The study did not require a formal approval from the ethics committee according to the Italian law since it was performed as a retrospective description of a clinical case in the context of normal clinical routines (art.1, leg. decree 211/2003). However, it was performed according to the guidelines of the Declaration of Helsinki. Data were previously anonymized, according to the requirements set by Italian Data protection Code (leg. Decree 196/ 2003).
The patient, a 52-year-old Caucasian female, was diagnosed with aHUS at the age of 26.
Genetic results disclosed the presence of a rare missense variant, in the complement C3 gene (S1063R), in association with a heterozygous nonsense mutation in the complement factor H (CFH) gene (E1172Stop), reported as pathogenic.
She presented two aHUS relapses at the age of 27 and 41 years. The first relapse required bilateral nephrectomy in order to achieve hematologic remission. At the age of 32, she received a kidney transplant from a standard criteria deceased donor. A flu vaccine triggered the second relapse. The patient was unsuccessfully treated with steroids and 5 sessions of plasma-exchange, with progressive graft loss. Then she started chronic hemodialysis treatment.
In October 2020, she presented with fever, diarrhea, headache and asthenia. SARS-CoV-2 infection was suspected and confirmed (PCR, throat swab); therefore, she was admitted to the SARS-CoV-2 Unit of the Polyclinic of Bari. At the admission, the chest X-ray was normal, and she showed an efficient respiratory profile with normal blood gas analysis results (pH: 7.44, pCO2: 36 mmHg, pO2: 77 mmHg, PO2/FiO2 = 376). Inflammatory markers were moderately increased (C-reactive protein 23.4 mg/l, D-dimer 235 ug/L and interleukin-6 50.6 pg/ml), showing a clinically mild form of SARS-CoV-2. Since the admission, the patient received azithromycin 500 mg per os (once daily for 2 weeks), i.v. dexamethasone (6 mg once daily for 16 days followed by a fast tapering) and s.c. enoxoparin (4000 UI/daily for 50 days).
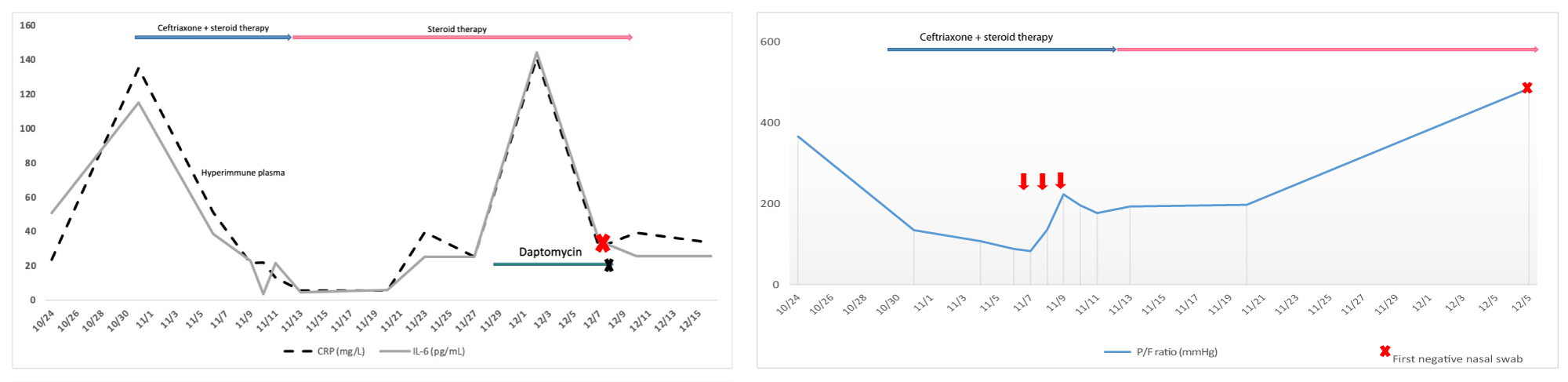
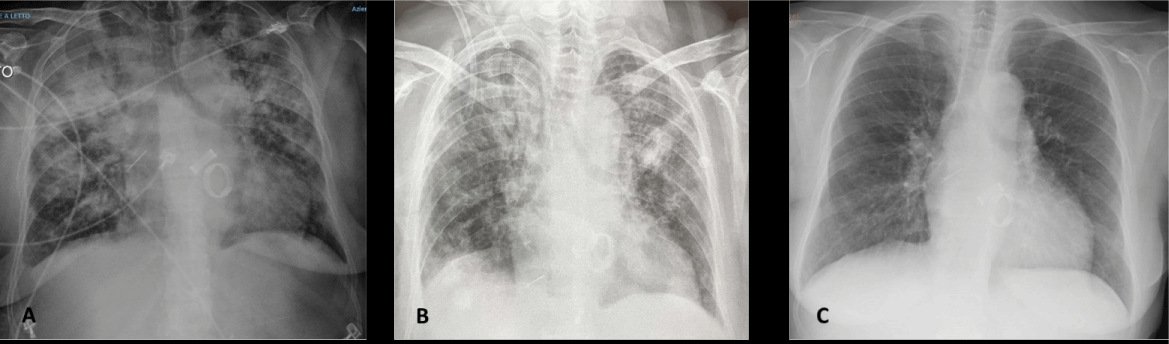
During the following two weeks, her clinical condition progressively worsened, as she presented dry cough, shortness of breath, and a substantial decrease in Oxygen Saturation down to 89%. The respiratory failure required Venturi masks (FiO2 60%), followed by Continuous Positive Airway Pressure (CPAP) with maximization of FiO2 (100%) and of Positive End Expiratory Pressure (PEEP) (10 cmH2O). Laboratory tests showed a severe increase of inflammatory markers (Figure 1A). Chest X-ray revealed signs of polysegmental bilateral viral pneumonia with multiple ground glass opacity areas (Figure 2A).
At the onset of respiratory symptoms, Ceftriaxone 2 g i.v. (once daily for 16 days) was added to prevent bacterial co-infection. Despite such treatment, the clinical worsening required intensive care unit (ICU) support. Therefore, we decided to start a rescue therapy with three doses of fresh frozen plasma (250 ml for each dose) obtained from a SARS-CoV-2 convalescent patient, according to the Tsunami Protocol. After the second plasma infusion, blood gas analysis revealed an improvement of PO2/FiO2 ratio: from 84, at the beginning of the therapy, to 224 on the 3rd day of plasma administration (Figure 1B). At the 3rd day of plasma infusion, Continuous Positive Airway Pressure (CPAP) parameters were reduced to FiO2 70% and PEEP 8 cmH2O and subsequent shifted to HFNC support with a gradually decrease of FiO2 since the 10th of November. The PO2 - FiO2 ratio showed a slightly decrease between the days 10th - 20th of November corresponding to a gradually ventilator weaning and the recovery of the spontaneous breathing.
Figure 1: A) Trend of inflammatory markers: CRP line blue; IL-6-line gray. The red arrows indicate the hyper-immune plasma administration (between 7th to 9th of November). The patient presents two distinct infection peaks. During the first peak bacterial super-infection was not detected and the X-ray confirmed a COVID-19 pneumonia. CRP and IL-6 showed a significant decrease after the plasma infusion until the 28/29th of November when the patient developed a bacterial infection of the central venous catheter. B) Trend of PO2-FiO2 ratio. The patient developed a severe and fast decrease of P/F ratio requiring a ventilatory support since the 4th of November (CPA PEEP 8 cmH20 FiO2 80% - 100%). Hyper-immune plasma was administrated between 7th to 9th of November. There was an improvement of PO2/FiO2 ratio: from 84, at the beginning of the therapy, to 224 on the 3rd day of plasma administration without other ventilation change. The PO2-FiO2 ratio showed a slightly decrease between the 10th - 20th of November corresponding to a gradually ventilator weaning and recovery of the spontaneous breathing. On the top of the pictures, the two lines represent the concomitant medications. Pink line: i.v. dexamethasone (6 mg once daily for 16 days followed by a fast tapering) which had been administered since the admission. Blue line: i.v. Ceftriaxone that was added when clinical symptoms worsened. The “x” represents the time of first negative nasopharyngeal swab for SARS-CoV-2.
Figure 2: A) Chest X-ray revealed signs of polysegmental bilateral viral pneumonia with multiple ground glass opacity areas (11/04/2020) B) Chest X-ray (11/09/2020) disclosed a decrease of the pleural effusion on the right. C) The chest X-ray (12/23/2020) revealed a complete resolution of SARS-CoV-2 -pneumonia.
Chest X-ray performed just one day after the last plasma administration, showed a slight reduction of the ground glass opacity areas at the mid-apical level of left lung. 48 hours after the completed immune plasma infusion cycle, the patient returned to our Unit given the respiratory and overall clinical improvement. Inflammatory markers showed a significant decrease after the plasma infusion and a durable change until the end of November when the patient developed a bacterial infection of the central venous catheter (Staphylococcus Epidermidis was detected in blood cultures). Therefore, the central venous catheter for hemodialysis was removed and daptomycin (7 mg/Kg every 48 h) was administered for 10 days with a complete clinical recovery.
During the first week after the end of plasma therapy a slightly decrease in platelet and hemoglobin was observed, associated with a reduction of C3 and C4, without signs of hemolysis (LDH: 288 UI/L, no schistocytes in the blood smear, bilirubin 0.44 mg/dl); therefore aHUS recurrence was excluded.
Inflammatory markers improved and the chest X-ray showed a resolution of SARS-CoV-2 pneumonia (Figure 2C). Hence, 55 days after the SARS-CoV-2 diagnosis, she was discharged.
Hyperimmune Plasma (HP) has been successfully used in several viral epidemics such as SARS, MERS, and Ebola [3]. Given this evidence, HP has also been administered to some SARS-CoV-2 patients. The World Health Organization (WHO) allowed the CP treatment for «serious diseases for which there are no effective pharmacological treatments». Nevertheless, the literature has showed contrasting results regarding the benefit of plasma therapy in SARS-CoV-2 patients and there is no recommendation on which type of patient HP should be administered. This case provide evidence that HP therapy can be considered as a rescue therapy in patients with aHUS history and moderate- to severe SARS-CoV-2 infection within 14 days of onset, maintaining a safety profile and without the risk of aHUS relapse.
Some reports showed the utility of HP therapy in SARS-CoV-2 patients, especially if it is administrated during the stage of viral replication [3]. The efficacy has been demonstrated mainly in sickly patients with a severe immunodeficiency, which had a relatively lower viral clearance and later antibody response [4].
Though aHUS patients are not immunodeficient, they seem to be exposed to higher risk of thrombotic event and recurrence of HUS [5].
Coronavirus 19 induces a direct endothelial damage and an alternative pathway complement (APC) activation through the SARS-CoV-2 spike surface protein [6]. The complement activation amplifies the endothelial injury, mainly in subjects with a genetic defect in the complement regulation, exposing aHUS patients with SARS-CoV-2 to a higher risk of microangiopathy. Therefore, the management of these sickly patients must be prompt to reduce the addi-tional complications triggered by complement activation. In literature, some cases [5,8] of aHUS patient who developed disease recurrence triggered by SARS-CoV-2 infection has been described. Only in one aHUS patient HP has been administered, during the worsening of COVID pneumonia, with successful outcome [9]. That patient did not require mechanical ventilation and did not have a known complement genetic variant neither aHUS recurrence history [9]. In contrast, our patient developed a severe form of SARS-CoV-2 pneumonia, requiring mechanical ventilation and ICU support. Considering the high susceptibility of our patient to HUS recurrence and the critically worsening respiratory function, we decided to administer HP as rescue therapy. Our patient had a good response to the HP treatment, which also showed a favorable safety profile. Moreover, she had no HUS recurrence, maybe because HP may reduce the viral replication.
In conclusion, our observation, although limited to a single patient, shows that HP can be considered as rescue therapy in patients with moderate-severe SARS-CoV-2 infection and aHUS history, and does not increase the risk of microangiopathy recurrence.
Author Contributions: E.D. Stea wrote the manuscript with support from F. Pesce. V. Pronzo collected the data and designed the figures. All authors provided critical feedback and helped shape the analysis and manuscript. L. Gesualdo supervised the project. Finally, all the authors define the therapy and the medical care of the patient during the job in the Nephrology COVID Unit of Bari.
Data availability statement: All data generated during this study are included in this article. Further enquiries can be directed to the corresponding author.
Disclosure: the authors declare no conflict of interest.
- Noris M, Benigni A, Remuzzi G. The case of complement activation in COVID-19 multiorgan impact. Kidney Int. 2020; 98: 314-322. PubMed: https://pubmed.ncbi.nlm.nih.gov/32461141/
- Zipfel PF, Wiech T, Stea ED, Skerka C. CFHR Gene Variations Provide Insights in the Pathogenesis of the Kidney Diseases Atypical Hemolytic Uremic Syndrome and C3 Glomerulopathy. J Am Soc Nephrol. 2020; 31: 241-256. PubMed: https://pubmed.ncbi.nlm.nih.gov/31980588/
- Petrungaro A, Quartarone E, Sciarrone P. Anti-SARS-CoV-2 hyperimmune plasma workflow. Transfus Apher Sci. 2020; 59: 102850. PubMed: https://pubmed.ncbi.nlm.nih.gov/32540345/
- Cusi MG, Conticini E, Gandolfo C, Anichini G, Savellini GG, et al. Hyperimmune plasma in three immuno-deficient patients affected by non-severe, prolonged COVID-19: a single-center experience. BMC Infect Dis. 2021; 21: 630. PubMed: https://pubmed.ncbi.nlm.nih.gov/34210259/
- Ville S, Le Bot S, Chapelet-Debout A, Blancho G, Fremeaux-Bacchi V, et al. Atypical HUS relapse triggered by COVID-19. Kidney Int. 2021; 99: 267-268. PubMed: https://pubmed.ncbi.nlm.nih.gov/33188793/
- Yu J, Yuan X, Chen H, Chaturvedi S, Braunstein EM, et al. Direct activation of the alternative complement pathway by SARS-CoV-2 spike proteins is blocked by factor D inhibition. Blood. 2020; 136: 2080-2089. PubMed: https://pubmed.ncbi.nlm.nih.gov/32877502/
- Maharaj N, Sankat S, Spann J, Goorachan S, Sookoo A. POS-041 Haemolytic Uremic Syndrome (HUS) with Covid-19 infection: 2 case reports. Is there a direct link? Kidney Int Rep. 2021; 6: S18–19. PubMed: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8049736/
- Jhaveri KD, Meir LR, Flores Chang BS, Parikh R, Wanchoo R, et al. Thrombotic microangiopathy in a patient with COVID-19. Kidney Int. 2020; 98: 509-512. PubMed: https://pubmed.ncbi.nlm.nih.gov/32525010/
- Trimarchi H, Gianserra R, Lampo M, Monkowski M, Lodolo J. Eculizumab, SARS-CoV-2 and atypical hemolytic uremic syndrome. Clin Kidney J. 2020; 13: 739-741. PubMed: https://pubmed.ncbi.nlm.nih.gov/33117528/