More Information
Submitted: February 02, 2024 | Approved: February 19, 2024 | Published: February 20, 2024
How to cite this article: Campana EB, Atti M. Efficiency, Effectiveness and Clinical Results of Extracorporeal Therapies in Non-Renal Settings: How are they to be evaluated? The Case of their Application in Liver Failure. J Clini Nephrol. 2024; 8: 008-016.
DOI: 10.29328/journal.jcn.1001120
Copyright License: © 2024 Campana EB, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: Extra blood purification therapies; Sorbents; Mass balance; Bilirubin; Liver failure
Efficiency, Effectiveness and Clinical Results of Extracorporeal Therapies in Non-Renal Settings: How are they to be evaluated? The Case of their Application in Liver Failure
Fausto Bruno Campana and Mauro Atti*
Aferetica Srl, Via Spartaco 10 – 40138, Bologna, Italy
*Address for Correspondence: Mauro Atti, Aferetica Srl, Via Spartaco 10 – 40138, Bologna, Italy, Email: [email protected]
There are various Extra Blood Purification Therapies (EBPTs) used in the context of critical care, including but not limited to Acute Kidney Injury (AKI). These therapies aim to remove toxins, inflammatory mediators, and excess fluids from the bloodstream. While some blood purification therapies were initially developed for renal support, they have been explored for use in other medical conditions as well, including liver pathologies and sepsis. Here is a brief explanation of some therapies such as MARS (Molecular Adsorbents Recirculating System), Prometheus, CPFA (Coupled Plasma Filtration Adsorption), PAP (Plasma Adsorption), and SPAD (Single-Pass Albumin Dialysis). Some of these therapies have entered clinical use, while others have faced challenges, such as negative evidence, poor purifying efficacy, or difficulties in practical use. The field of extracorporeal liver support is dynamic, with ongoing developments aimed at improving the effectiveness and practicality of these therapies. Sorbents mark the latest frontiers in blood purification to remove various toxic molecules, with specific emphasis on the modulation of bilirubin and other substances in critically ill patients suffering from liver failure. In the above-mentioned pathologies, substances may be continuously generated within the body, and Mass Balance is the only valuable tool for distinguishing between generation and removal processes. The effectiveness of sorbents in removing bilirubin and bile acids, as demonstrated in both in vitro and in vivo studies, distinguishes them and shows their superiority over traditional liver cleansing methods, such as CPFA, PAP, SPAD, Prometheus, and MARS.
Various adjuvant Extra Blood Purification Therapies (EBPTs) have been developed to modulate toxins and uncontrolled substances in different pathologies. Some EBPTs derive directly from dialysis techniques. High-volume hemofiltration (HVHF) is described as a modification of continuous renal replacement therapy (RRT) in hemofiltration mode. The focus is on using high fluid volumes for filtration to enhance substance removal. Hemofiltration with Highly Adsorbing Membranes involves using highly adsorbing membranes during hemofiltration to enhance the removal of substances from the blood. High Cut-Off membranes are used to expand the range of middle molecular weight molecule removal and the membrane cut-off value is increased. This modification aims to improve the efficiency of the blood purification process. Other EBPTs come directly from Plasma Exchange (PEX). The PEX technique has been evaluated in the context of liver failure. The goal is to remove or exchange plasma components to address specific pathologies. Unfortunately, PEX does not always have its advantages. In a prospective, randomized, controlled, multicenter trial, Larsen, et al. [1] randomly assigned 182 patients with ALF to receive either standard medical therapy (SMT;90 patients) or SMT plus High-volume plasma exchange (HVP) for three days (92 patients). 1,5 l of plasma was exchanged and the total volume exchanged was 15% of body weight or about 8-12 l per day per procedure. The data showed that the main effect of HVP on survival was achieved in the patients not undergoing emergency liver transplantation or were either not transplant candidates or not transplanted. Treatment with HVP improved outcomes in patients with ALF by increasing liver transplant-free survival. This was attributable to the attenuation of innate immune activation and the amelioration of multi-organ dysfunction. The negative side of this study is that PEX exchanged 12 l of plasma (meaning up to 4 times the usual value of patient plasma) which is unbearable for patients, in clinical practice. Furthermore, PEX requires a plasma filter for plasma separation and high quantities of albumin solution/fresh frozen plasma, with the risks associated with this practice as it causes the removal of all plasma components. Derived directly from PEX, Cascade filtration is designed to selectively remove middle molecules while retaining smaller ones such as trace elements, vitamins, and medications. This is achieved by combining one plasma filter and a hemofilter with distinct cut-off values, allowing for targeted removal. Coupled Plasma Filtration and Adsorption (CPFA) is described as an extracorporeal therapy where plasma is separated from the blood at the start of the circuit. Plasma then runs slowly through a sorbent, facilitating the removal of certain substances. Hemoperfusion involves the patient’s blood coming into contact with adsorbing materials. Molecules can adhere to these materials through various interactions, such as van der Waals, electrostatic, and hydrophobic interactions [2].
Liver failure
Among the toxins, the removal of bilirubin, bile acids, and ammonium in critically ill patients with liver failure is considered an effective strategy to improve organ dysfunction and the overall health status of these patients. Here, in particular, we discuss the parallel pathway existing between acute forms of liver dysfunction and failure and the pathophysiological features seen in sepsis. It is highlighted that both conditions involve a dysregulated inflammatory response. In fact, Acute liver failure shares significant similarities with septic shock and there is a complex interplay of systemic inflammation, immune dysfunction, and organ failure in acute liver failure. Also in Acute Liver Failure, a massive and uncontrolled activation of the systemic inflammatory response, paralleled by an equally uncontrolled anti-inflammatory response, results in progressive MODS. In ACLF, circulating cytokines are related to prognosis in different stages of cirrhosis, and a stronger anti-inflammatory response was associated with progression to death. Patients with ACLF showed systemic inflammation and systemic circulatory dysfunction, with higher levels of various inflammatory cytokines than the patients without ACLF. These data support systemic inflammation as the primary driver of ACLF in cirrhosis [3-5]. In the context of liver dysfunction, this dysregulation of the inflammatory response is considered the basis for the rationale behind using hemadsorption. Hepatic dysfunction refers to a condition where the liver experiences impairment in its main functions, which include detoxification, synthesis, and regulation. The detoxification function is particularly crucial, as it involves the metabolism of various molecules. When this function is compromised, certain molecules accumulate in the systemic circulation, leading to metabolic and biochemical alterations. To address hepatic dysfunction, Extra Corporeal Liver Support (ECLS) systems have been developed. These systems aim to support the liver’s detoxification function by clearing the blood from hepatic toxic molecules. The goal is to bridge the patient either to orthotopic liver transplantation or to functional recovery. ECLS is indicated in various conditions such as acute liver failure (ALF), acute-on-chronic liver failure (ACLF), primary non-function (PNF) after liver transplantation, post-hepatectomy liver failure (PHLF), or in patients with intractable pruritus [6,7].
Extra blood purification therapies
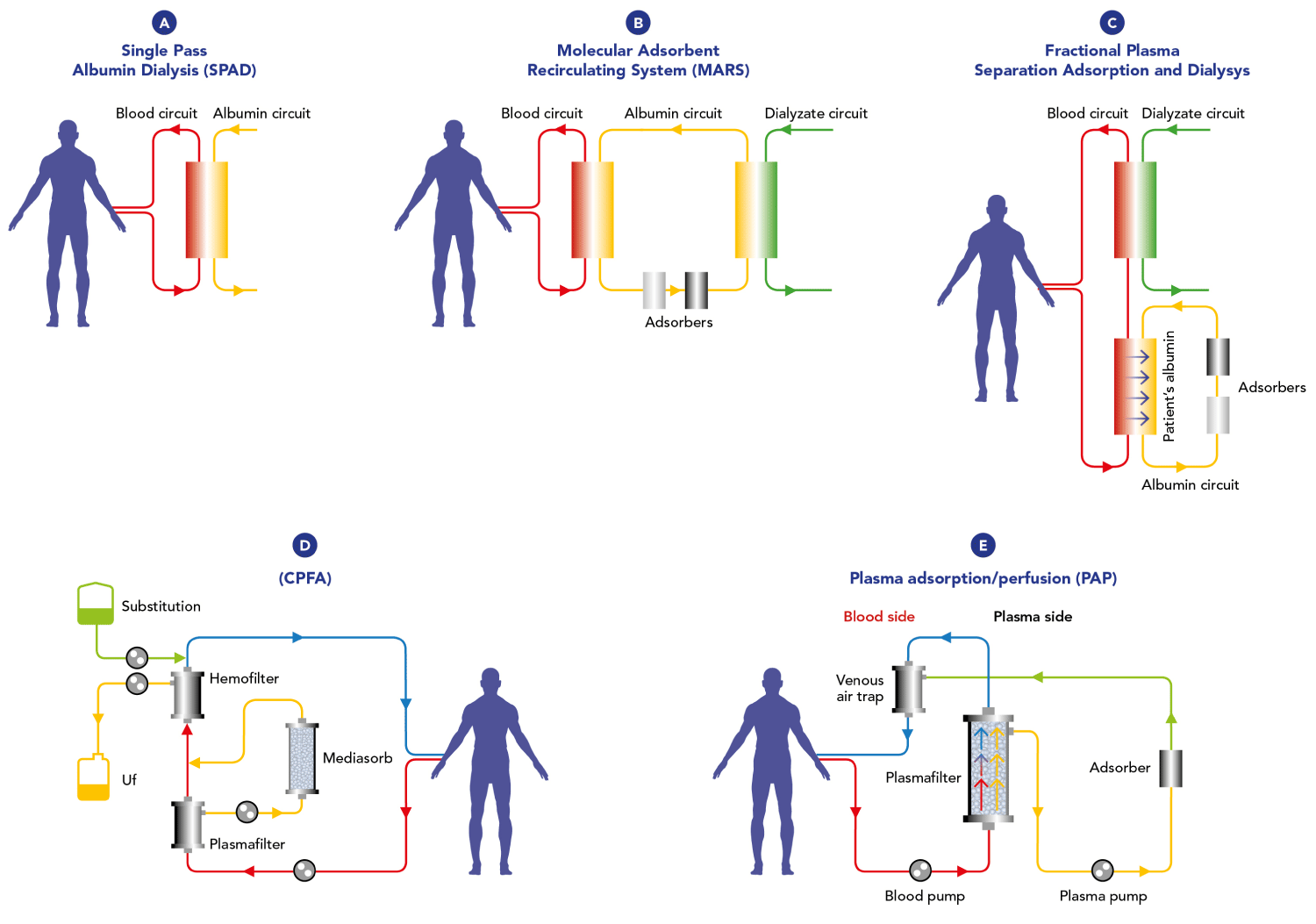
Different EBPTs have been proposed for liver failure, specifically involving bilirubin modulation (Figure 1). Some of them have entered clinical use, while some have been largely abandoned. This could be due to various reasons, including a lack of evidence supporting their effectiveness. Poor purifying efficacy and difficulty in use are mentioned as specific challenges that have led to the abandonment of certain therapies. These challenges could relate to the limitations in effectively removing toxins, including bilirubin, from the bloodstream. MARS consists of a double circuit: the blood circuit and the albumin circuit, which are separated from each other by an impermeable albumin membrane whose pores are about 50 kDa. Prometheus combines plasma separation and absorption. It consists of a double circuit: the blood circuit and the plasma circuit are separated by a membrane whose pores of about 250 kDa are permeable to albumin. SPAD is based on hemodialysis and hemofiltration principles and is the simplest system, as it can be implemented with a standard CRRT device. The patient’s blood passes through a standard high-flow membrane impermeable to albumin, and dialyses counter-currently with a solution containing 2-5% albumin that is discarded after a single passage Coupled Plasma Filtration Adsorption (CPFA) is a technique that separates the plasma from the blood by means of a plasma filter. The plasma is then passed through a synthetic resin cartridge and returned to the blood. A second blood filter can be then used to remove excess fluid and small molecular weight toxins where necessary, as in the case of acute renal failure. PAP (very similar to CPFA) consists of plasma separation from the blood by a plasma filter (material effective surface area 0,45m², pore size 0.5/0.7 µm, sieving coefficient of albumin) and then passage through a sorbent cartridge at a flow rate of 40 mL/min. All these techniques have produced contradictory results, and technical complexity, and have limited treatment duration, limited hepatic toxin removal, and difficulty in albumin-bound toxin removal [8-11].
Hemadsorption
Among the EBPTs, hemadsorption by sorbents marks the latest frontier [12]. Hemadsorption involves placing sorbents contained in cartridges in direct contact with the blood via an extracorporeal circuit. This process is designed to remove toxins (e.g. bilirubin, myoglobin, drugs) and inflammatory mediators from the blood. To show that, Gruda, et al. [13] have added purified proteins to whole blood at clinically relevant concentrations and recirculated through a device filled with hemoadsorbent polymer beads or control (no bead) device in vitro: hemadsorption through porous polymer bead devices have reduced the levels of a broad spectrum of cytokines (MIP1-α, IL-6, and IFN-γ, TNF-α DAMPS (C5a, HMGB-1, procalcitonin, and S100-A8), PAMPS (α-toxin, SpeB, and TSST-1) and mycotoxins (aflatoxin, T-2 toxin). Initially designed for the modulation of cytokines, it has proven to be effective in bilirubin and bile acid removal as well. In fact, in an in Vitro study, Gemelli, et al. [14] demonstrated with the same sorbent the capability to efficiently remove bilirubin over 24 hours, with minimal albumin loss and no detectable bilirubin release from the charged resin. In a patient with liver dysfunction, Buttner, et al. [15] performed the measurement of pre- and post-adsorber values, showing that also the removal of ammonia is directly attributable to the adsorber, although this is a debated concept [16]. The rationale behind using adsorption therapy is to restore the immune balance, encompassing both pro-inflammatory and anti-inflammatory responses, as a cytokine storm is characterized by an excessive release of pro- and anti-inflammatory cytokines, which can lead to severe systemic inflammation. Extracorporeal hemadsorption is commonly employed by intensive care specialists to prevent or counteract the effects of a cytokine storm, primarily in conditions such as septic shock and other forms of vasoplegic shock [17].
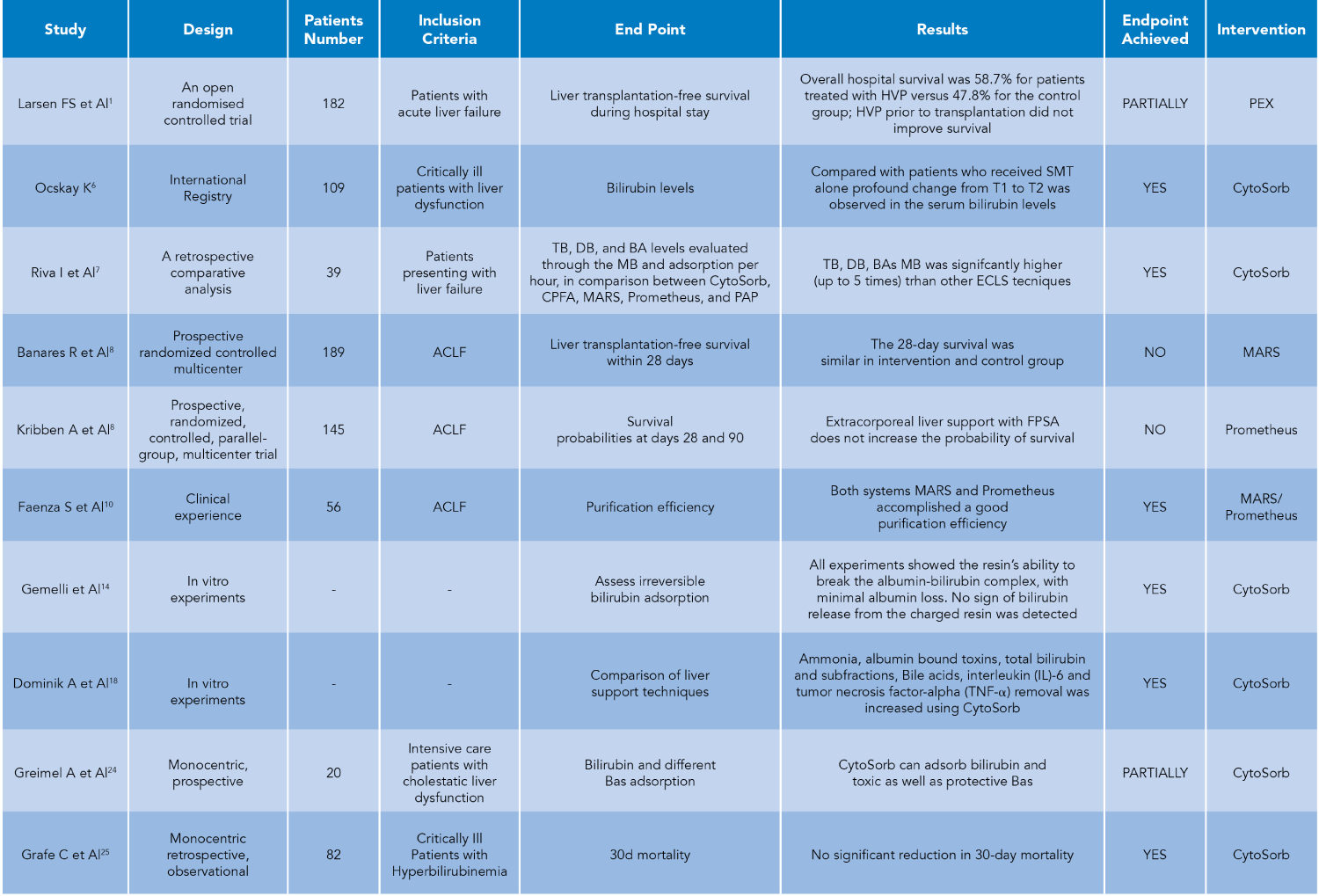
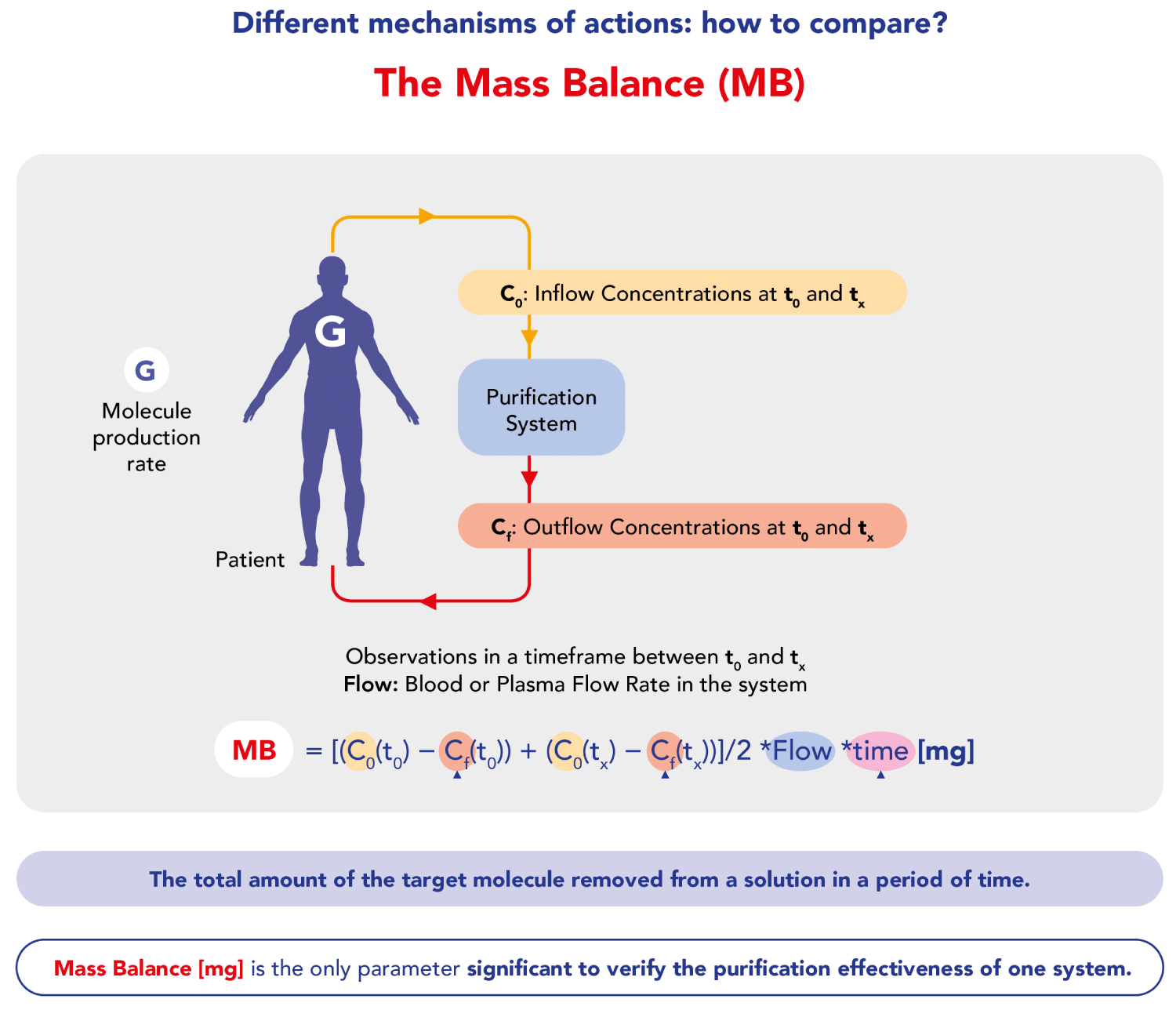
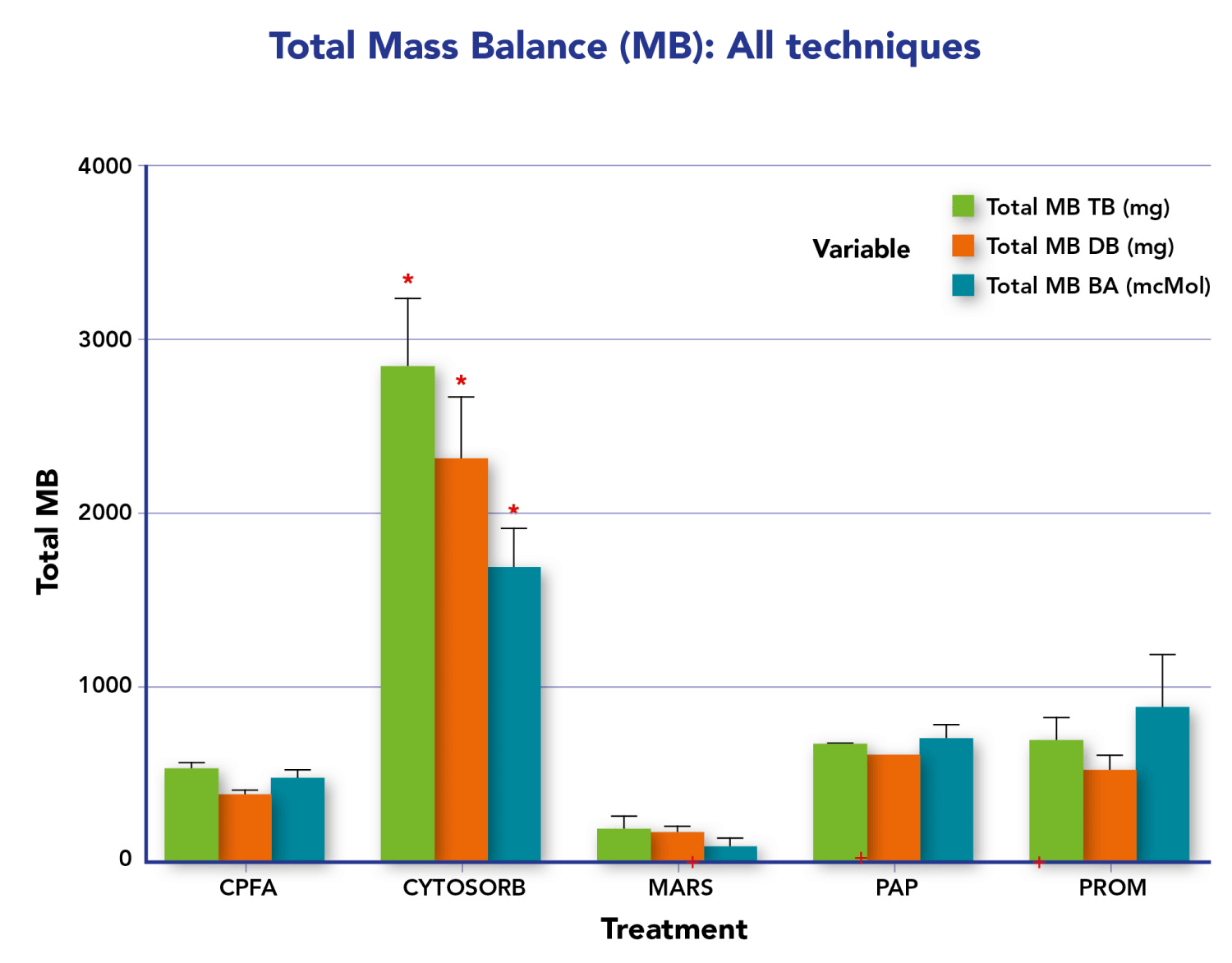
Here we summarize various studies (Table 1) evaluating the effectiveness, efficiency, and clinical results of extracorporeal therapies, focusing on sorbents in non-renal settings, particularly on liver failure, highlighting that Mass Balance (MB) is the only valid parameter for assessing the purification effectiveness of extracorporeal systems, as it is not affected by endogenous generation (Figure 2). Gemelli, et al. [14] have demonstrated sorbent’s capability to efficiently remove bilirubin over 24 hours, with minimal albumin loss and no detectable bilirubin release from the charged resin. The Authors conducted four in vitro experiments with different albumin-bilirubin solutions, showing massive and irreversible adsorption of bilirubin by the resin. Efficacy in detaching unconjugated bilirubin from albumin and continuous adsorption over 24 hours were highlighted. In addition to what we have said, bear in mind that also in dialysis techniques mass balance is crucial in understanding and comparing various methods. Mass balance allows us to evaluate the effectiveness of a purifying therapy by distinguishing the components of equilibrium between the input and output of solutes in the body during the dialysis process. Normalization of the distribution’s volume, the apparent space in the body available to contain a solute, suggests how to adjust the mass balance to obtain the right Kt/V, the parameter universally used in dialysis treatments. This normalization helps in comparing different dialysis methods more effectively by accounting for variations in the distribution of substances within the body. Therefore, applying mass balance and normalization techniques ensures accurate comparisons and a better understanding of the underlying processes. Among the sorbents, CytoSorb® is a notable technology and has been described as the most investigated and clinically established procedure among the presented technologies. It is specifically approved for the remodulation of pro and anti-inflammatory cytokines, as well as bilirubin and myoglobin, in addition to the removal of certain medications like ticagrelor and rivaroxaban. This suggests that CytoSorb® is recognized for its efficacy in addressing a range of inflammatory and toxic substances in the blood during extracorporeal blood purification therapies [18-41]. Furthermore, it is the only certified sorbent that can remove bilirubin (as well as cytokines) directly from the whole blood [42], without any need for plasma separation as indicated in Jafron CE [43]. This is confirmed by Riva, et al. [7] in a published in vivo study. The Authors indicate the superior adsorption of the sorbent in terms of Mass Balance (MB) compared to other extracorporeal techniques (CPFA, MARS, Prometheus, PAP). The study showed significantly higher adsorption of Total Bilirubin (TB), Direct Bilirubin (DB), and Bile Acids (BAs) with sorbent, with an average 5-fold higher MB for CytoSorb sorbent compared to other methods (Figure 3). These in vivo results are consistent with the results of in vitro studies conducted by Stange’s group [18]. Furthermore, the study by Ocksay, et al. [6] in a 1434-patient registry, specifically in a subgroup of 109 liver failure patients, confirms that hemadsorption significantly removes bilirubin from the blood. This finding is notable as previous studies with small sample sizes or retrospective data had shown similar effects. Many cases reports and case series results have been collected by Turan, et al. [44], who conducted a systematic review and meta-analysis that aimed to assess the evidence on clinical outcomes following hemadsorption therapy. The systematic review and meta-analysis provide a comprehensive overview of existing studies. The search covered six electronic databases and identified 30 eligible publications from 2011 to 2023, involving a total of 335 patients with liver dysfunction related to acute critical illness. The analysis of individual cases indicated a significant reduction in levels of aspartate transaminase and vasopressor needs, as well as a trend towards lower levels of total bilirubin, alanine transaminase, C-reactive protein, and creatinine. Pooled data also showed a significant reduction in total bilirubin, confirming the direct relationship between liver toxin decrease and function improvement. Overall, the findings suggest that the use of hemadsorption for critically ill patients with acute liver dysfunction or failure appears to be safe and shows a trend toward improved liver function after therapy. Therefore, thanks to efficacy, safety profile, and easy handling, sorbents represent a clear advantage over the older techniques cited above.
Table 1: Comparative Analysis of Extracorporeal Sorbent Therapies in Liver Failure
Figure 1: Extra blood purification therapies for liver failure. A(SPAD); B(MARS);C(Prometheus);D(CPFA);E(PAP).
Figure 2: Mass Balance rationale and formula.
Figure 3: TB, DB and BA Total MB regarding all the techniques comparison for the overall treatments (Kruskalis-wallis). *Total MB – TB, DB, BA: p<0.05 CYTOSORB vs.CPFA, MARS and PROM; +Limited number of samples PAP (n = 2), MARS (n = 3), PROM (n = 5).
On the other hand, when bilirubin and BA removal are not measured by Mass Balance, but with systemic Rate Removal or percentage decrease, the results could be misleading. For example, the study by Greimel, et al. [45], titled “Extracorporeal adsorption of protective and toxic bile acids and bilirubin in patients with cholestatic liver dysfunction: a prospective study” is limited as the Authors did not measure Mass Balance (MB), which is the sole parameter for assessing the purification effectiveness of extracorporeal systems. MB accounts for the total amount of a molecule removed from a solution, offering an accurate reflection of the system’s performance. The formula for calculating MB involves pre- and post-removal concentrations at various time intervals (C0 and Cf) and the plasma flow rate (Qplasma).
MB [mg] = [(C0 (t0) −Cf (t0)) + (C0 (tx) −Cf (tx))] ∕ 2 × Qplasma × tx)
Last but not least, the study by Grafe, et al. [46] on the correlation between bilirubin elimination with CytoSorb® and mortality in critically ill patients with hyperbilirubinemia raises important considerations about the challenges of using mortality as a primary endpoint, the multifactorial nature of liver failure, and the need for standardized protocols and measurements in studies involving blood purification techniques. Concerns about using mortality as the primary endpoint in critically ill patients are well-founded. The variability in sepsis phenotypes and the complexity of critical conditions can make it challenging to attribute outcomes solely to one specific intervention. In fact, if using mortality as an outcome measure in studies involving critically ill patients is limiting, this is also particularly true for those with Acute Chronic Liver Failure (ACLF) and Acute Liver Failure (ALF), where the only chance of survival is liver transplantation. Mid- and long-term mortality may not be the most relevant or informative outcome to be studied in these patients. In cases where a life-saving intervention, such as liver transplantation, can significantly impact survival, focusing solely on mortality might not capture the nuances of treatment effectiveness or the overall patient experience.
Prospects
Researchers could consider other outcome measures that reflect not only survival but also quality of life, functional status, and other relevant factors for patients with ACLF or ALF. These could include parameters like post-transplant complications, graft function, and long-term morbidity. It is crucial that researchers select outcome measures that align with the clinical context and goals of the study, ensuring that the findings are meaningful and applicable to the specific patient population under investigation. Focusing on practical endpoints, as we suggest, such as improvements in clinical tests, liver function, and overall patient recovery, may provide a more comprehensive understanding of the intervention’s efficacy. Acknowledging the multifactorial nature of the liver failure is crucial as well. Addressing such complexity often requires a holistic approach that considers the underlying causes, management of symptoms, and supportive care. Highlighting the need for studies to focus on practical endpoints beyond mortality is a valid perspective. The lack of a standardized protocol for the sorbent administration and the absence of measurements for bilirubin quantity removed both raise concerns about the reproducibility and effectiveness of the intervention. Variability in treatment approaches could impact outcomes and, without specific measurements, it becomes challenging to assess the true impact of sorbent on bilirubin levels. Furthermore, the potential disregard of local bilirubin generation when relying on systemic measurements alone is important. Local effects may not be fully captured through systemic assessments, and understanding the dynamics of bilirubin removal at the site of action is crucial for a comprehensive evaluation of the sorbent’s impact. Our concerns highlight the need for future studies to employ standardized protocols, consider practical endpoints beyond mortality, and integrate comprehensive measurements to assess the effectiveness of blood purification techniques, such as sorbent in critically ill patients with hyperbilirubinemia. This collaborative and critical review process contributes to the ongoing improvement in research methodologies and the development of more effective interventions in critical care settings.
The use of extracorporeal hemadsorption in liver dysfunction is grounded in the similarities between the dysregulated inflammatory response observed in acute liver dysfunction and that seen in sepsis. By addressing the inflammatory component, hemadsorption aims to mitigate the effects of the dysregulated immune response, providing a potential therapeutic approach in the context of liver dysfunction. Therefore, the challenge is to determine the most effective method for extracorporeal liver support, highlighting the exploration of new techniques, particularly hemadsorption, as a potential avenue for effective toxin removal, such as bilirubin, from the bloodstream in the context of liver dysfunction. The assessment of the removal efficacy of Extra Blood Purification Therapies (sorbents in particular) is contingent upon performing Mass Balance, as other measurements are influenced by endogenous generation. Considering the fact that efficiency is the measure of accomplishing a task with minimal wasted resources, including time, money, and effort, while effectiveness gauges the degree to which an action or process successfully produces the desired outcome, and bearing in mind the results achieved hitherto, the easy handling, the timing, the safety and pharmacoeconomic factors, then we can conclude that sorbents have clearly shown benefits in improving patient outcomes by mitigating the harmful effects of excessive inflammation and by removing toxins and drugs.
Conflict of interest: Fausto Bruno Campana and Mauro Atti are both employed by Aferetica Srl.
- Larsen FS, Schmidt LE, Bernsmeier C, Rasmussen A, Isoniemi H, Patel VC, Triantafyllou E, Bernal W, Auzinger G, Shawcross D, Eefsen M, Bjerring PN, Clemmesen JO, Hockerstedt K, Frederiksen HJ, Hansen BA, Antoniades CG, Wendon J. High-volume plasma exchange in patients with acute liver failure: An open randomised controlled trial. J Hepatol. 2016 Jan;64(1):69-78. doi: 10.1016/j.jhep.2015.08.018. Epub 2015 Aug 29. PMID: 26325537.
- Girardot T, Schneider A, Rimmelé T. Blood Purification Techniques for Sepsis and Septic AKI. Semin Nephrol. 2019 Sep;39(5):505-514. doi: 10.1016/j.semnephrol.2019.06.010. PMID: 31514914.
- Antoniades CG, Berry PA, Wendon JA, Vergani D. The importance of immune dysfunction in determining outcome in acute liver failure. J Hepatol. 2008 Nov;49(5):845-61. doi: 10.1016/j.jhep.2008.08.009. Epub 2008 Aug 21. PMID: 18801592.
- Fischer J, Silva TE, Soares E Silva PE, Colombo BS, Silva MC, Wildner LM, Bazzo ML, Rateke EC, Frode TS, Mello SV, Rosa JS, Dantas-Correa EB, Narciso-Schiavon JL, Schiavon LL. From stable disease to acute-on-chronic liver failure: Circulating cytokines are related to prognosis in different stages of cirrhosis. Cytokine. 2017 Mar;91:162-169. doi: 10.1016/j.cyto.2016.12.017. Epub 2017 Jan 9. PMID: 28082235.
- Clària J, Stauber RE, Coenraad MJ, Moreau R, Jalan R, Pavesi M, Amorós À, Titos E, Alcaraz-Quiles J, Oettl K, Morales-Ruiz M, Angeli P, Domenicali M, Alessandria C, Gerbes A, Wendon J, Nevens F, Trebicka J, Laleman W, Saliba F, Welzel TM, Albillos A, Gustot T, Benten D, Durand F, Ginès P, Bernardi M, Arroyo V; CANONIC Study Investigators of the EASL-CLIF Consortium and the European Foundation for the Study of Chronic Liver Failure (EF-CLIF). Systemic inflammation in decompensated cirrhosis: Characterization and role in acute-on-chronic liver failure. Hepatology. 2016 Oct;64(4):1249-64. doi: 10.1002/hep.28740. Epub 2016 Aug 25. PMID: 27483394.
- Ocskay K, Tomescu D, Faltlhauser A, Jacob D, Friesecke S, Malbrain M, Kogelmann K, Bogdanski R, Bach F, Fritz H, Hartjes A, Kortgen A, Soukup J, Utzolino S, van Tellingen M, Träger K, Schumacher U, Brunkhorst FM, Molnar Z. Hemoadsorption in 'Liver Indication'-Analysis of 109 Patients' Data from the CytoSorb International Registry. J Clin Med. 2021 Nov 5; 10(21):5182. doi: 10.3390/jcm10215182. PMID: 34768702; PMCID: PMC8584981.
- Riva I, Marino A, Valetti TM, Marchesi G, Fabretti F. Extracorporeal liver support techniques: a comparison. J Artif Organs. 2023 Jun 19. doi: 10.1007/s10047-023-01409-9. Epub ahead of print. PMID: 37335451.
- Bañares R, Nevens F, Larsen FS, Jalan R, Albillos A, Dollinger M, Saliba F, Sauerbruch T, Klammt S, Ockenga J, Pares A, Wendon J, Brünnler T, Kramer L, Mathurin P, de la Mata M, Gasbarrini A, Müllhaupt B, Wilmer A, Laleman W, Eefsen M, Sen S, Zipprich A, Tenorio T, Pavesi M, Schmidt HH, Mitzner S, Williams R, Arroyo V; RELIEF study group. Extracorporeal albumin dialysis with the molecular adsorbent recirculating system in acute-on-chronic liver failure: the RELIEF trial. Hepatology. 2013 Mar;57(3):1153-62. doi: 10.1002/hep.26185. Epub 2013 Feb 15. PMID: 23213075.
- Kribben A, Gerken G, Haag S, Herget-Rosenthal S, Treichel U, Betz C, Sarrazin C, Hoste E, Van Vlierberghe H, Escorsell A, Hafer C, Schreiner O, Galle PR, Mancini E, Caraceni P, Karvellas CJ, Salmhofer H, Knotek M, Ginès P, Kozik-Jaromin J, Rifai K; HELIOS Study Group. Effects of fractionated plasma separation and adsorption on survival in patients with acute-on-chronic liver failure. Gastroenterology. 2012 Apr;142(4):782-789.e3. doi: 10.1053/j.gastro.2011.12.056. Epub 2012 Jan 13. PMID: 22248661.
- Faenza S, Baraldi O, Bernardi M, Bolondi L, Coli L, Cucchetti A, Donati G, Gozzetti F, Lauro A, Mancini E, Pinna AD, Piscaglia F, Rasciti L, Ravaioli M, Ruggeri G, Santoro A, Stefoni S. Mars and Prometheus: our clinical experience in acute chronic liver failure. Transplant Proc. 2008 May;40(4):1169-71. doi: 10.1016/j.transproceed.2008.03.069. PMID: 18555140.
- De Gasperi A. Il trattamento dell'patite acuta fulminante: trattamento intensivo, sostituzione artificiale, trapianto epatico [Fulminant liver failure: intensive care, extracorporeal treatment and liver transplantation]. G Ital Nefrol. 2006 May-Jun;23 Suppl 36:S61-8. Italian. Erratum in: G Ital Nefrol. 2007 Nov-Dec;24(6):628-9. PMID: 17068731.
- Zarbock A, Nadim MK, Pickkers P, Gomez H, Bell S, Joannidis M, Kashani K, Koyner JL, Pannu N, Meersch M, Reis T, Rimmelé T, Bagshaw SM, Bellomo R, Cantaluppi V, Deep A, De Rosa S, Perez-Fernandez X, Husain-Syed F, Kane-Gill SL, Kelly Y, Mehta RL, Murray PT, Ostermann M, Prowle J, Ricci Z, See EJ, Schneider A, Soranno DE, Tolwani A, Villa G, Ronco C, Forni LG. Sepsis-associated acute kidney injury: consensus report of the 28th Acute Disease Quality Initiative workgroup. Nat Rev Nephrol. 2023 Jun;19(6):401-417. doi: 10.1038/s41581-023-00683-3. Epub 2023 Feb 23. PMID: 36823168.
- Gruda MC, Ruggeberg KG, O'Sullivan P, Guliashvili T, Scheirer AR, Golobish TD, Capponi VJ, Chan PP. Broad adsorption of sepsis-related PAMP and DAMP molecules, mycotoxins, and cytokines from whole blood using CytoSorb® sorbent porous polymer beads. PLoS One. 2018 Jan 25;13(1):e0191676. doi: 10.1371/journal.pone.0191676. PMID: 29370247; PMCID: PMC5784931.
- Gemelli C, Cuoghi A, Magnani S, Atti M, Ricci D, Siniscalchi A, Mancini E, Faenza S. Removal of Bilirubin with a New Adsorbent System: In Vitro Kinetics. Blood Purif. 2019; 47(1-3):10-15. doi: 10.1159/000492378. Epub 2018 Sep 14. PMID: 30219813.
- Büttner S, Patyna S, Koch B, Finkelmeier F, Geiger H, Sarrazin C, Farnik H. Application of Hemoadsorption in a Case of Liver Cirrhosis and Alcohol-Related Steatohepatitis with Preexisting Hepatitis C Infection. Blood Purif. 2017;44(1):30-31. doi: 10.1159/000455064. Epub 2017 Feb 25. PMID: 28237980.
- Liebchen U, Paal M, Gräfe C, Zoller M, Scharf C; Cyto-SOLVE Study Group. The cytokine adsorber Cytosorb® does not reduce ammonia concentrations in critically ill patients with liver failure. Intensive Care Med. 2023 Mar;49(3):360-362. doi: 10.1007/s00134-023-06998-w. Epub 2023 Feb 10. PMID: 36763124; PMCID: PMC9998587.
- Mehta Y, Paul R, Ansari AS, Banerjee T, Gunaydin S, Nassiri AA, Pappalardo F, Premužić V, Sathe P, Singh V, Vela ER. Extracorporeal blood purification strategies in sepsis and septic shock: An insight into recent advancements. World J Crit Care Med. 2023 Mar 9;12(2):71-88. doi: 10.5492/wjccm.v12.i2.71. PMID: 37034019; PMCID: PMC10075046.
- Dominik A, Stange J. Similarities, Differences, and Potential Synergies in the Mechanism of Action of Albumin Dialysis Using the MARS Albumin Dialysis Device and the CytoSorb Hemoperfusion Device in the Treatment of Liver Failure. Blood Purif. 2021;50(1):119-128. doi: 10.1159/000508810. Epub 2020 Jul 2. PMID: 32615564.
- Faenza S. Removal of bilirubin with a new adsorbent system: in vitro kinetics, 36th International Symposium on Intensive Care and Emergency Medicine : Brussels, Belgium. 15-18 March 2016. Crit Care. 2016 Apr 20;20(Suppl 2):94. doi: 10.1186/s13054-016-1208-6. Erratum in: Crit Care. 2016 Oct 24;20:347. PMID: 27885969; PMCID: PMC5493079.
- Jansen A, Waalders NJB, van Lier DPT, Kox M, Pickkers P. CytoSorb hemoperfusion markedly attenuates circulating cytokine concentrations during systemic inflammation in humans in vivo. Crit Care. 2023 Mar 21;27(1):117. doi: 10.1186/s13054-023-04391-z. PMID: 36945034; PMCID: PMC10029173.
- Mitzner S, Kogelmann K, Ince C, Molnár Z, Ferrer R, Nierhaus A. Adjunctive Hemoadsorption Therapy with CytoSorb in Patients with Septic/Vasoplegic Shock: A Best Practice Consensus Statement. J Clin Med. 2023 Nov 21;12(23):7199. doi: 10.3390/jcm12237199. PMID: 38068250; PMCID: PMC10707447.
- Baroni S, Melegari G, Brugioni L, Gualdi E, Barbieri A, Bertellini E. First experiences of hemoadsorption in donation after circulatory death. Clin Transplant. 2020 Jun;34(6):e13874. doi: 10.1111/ctr.13874. Epub 2020 Apr 27. PMID: 32339334.
- Baroni S, Marudi A, Rinaldi S, Ghedini S, Magistri P, Piero Guerrini G, Olivieri T, Dallai C, Talamonti M, Maccieri J, Benedetto FD, Bertellini E. Cytokine mass balance levels in donation after circulatory death donors using hemoadsorption: Case series report. Int J Artif Organs. 2022 Jul;45(7):642-646. doi: 10.1177/03913988221091288. Epub 2022 Apr 15. PMID: 35426347.
- Calabrò MG, Febres D, Recca G, Lembo R, Fominskiy E, Scandroglio AM, Zangrillo A, Pappalardo F. Blood Purification With CytoSorb in Critically Ill Patients: Single-Center Preliminary Experience. Artif Organs. 2019 Feb;43(2):189-194. doi: 10.1111/aor.13327. Epub 2018 Aug 29. PMID: 30156308.
- Bottari G, Murciano M, Merli P, Bracaglia C, Guzzo I, Stoppa F, Pardeo M, Nunziata J, Del Bufalo F, Genuini L, De Benedetti F, Locatelli F, Cecchetti C. Hemoperfusion with CytoSorb to Manage Multiorgan Dysfunction in the Spectrum of Hemophagocytic Lymphohistiocytosis Syndrome in Critically Ill Children. Blood Purif. 2022;51(5):417-424. doi: 10.1159/000517471. Epub 2021 Aug 3. PMID: 34344006.
- Zanini V, Borghi A, Vetrugno L, Cherchi V, Baccarani U, Bove T. Blood Purification After Liver Transplantation Could Be a Useful Choice? Blood Purif. 2019; 47 Suppl 4:1. doi: 10.1159/000500178. Epub 2019 May 6. PMID: 31170707.
- D’Arezzo M, Grandinetti V, Freddi P, Sagripanti S, Ricciatti AM, Moretti I, Brigante F, Cerruti E, Ranghino A. High Cut-Off Continuous Veno-Venous Hemodialysis Associated with Hemoadsorption Effectively Remove Bilirubin and Contribute to Prevent Hyperbilirubinemia Induced-Acute Kidney Injury. A Single Center Experience Blood Purif. 2019; 47 Suppl 4:1. doi: 10.1159/000500178. Epub 2019 May 6. PMID: 31170707.
- Marco G, Leonardo C, Rosalia DB, Raffaella R, Francesco V, Giuseppe M, Eliana V, Davide V, Katia M, Valeria T, Nicola S, Marta G. Cottone Santina Successful Treatment of Bilirubin Nephropathy by CytoSorb Hemodialysis Blood Purif. 2019; 47 Suppl 4:1. doi: 10.1159/000500178. Epub 2019 May 6. PMID: 31170707.
- Frattari A, D’Andrea E, Talamazzi S, Cacciatore P, Parruti G, Campanella M, Zocaro R. Bridging to Transplant a Patient with Acute-onChronic Hepatic Failure: The Role of CytoSorb Haemoadsorbtion. Blood Purif. 2019; 47 Suppl 4:1. doi: 10.1159/000500178. Epub 2019 May 6. PMID: 31170707.
- Report MT, Cazzato D, Gemma MG, Viola F, Caccetta A, Accogli V, Montinaro T. Pellis CytoSorb as an Organ Support Therapy During Acute Liver Failure: A Case Blood Purif. 2019; 47 Suppl 4:1. doi: 10.1159/000500178. Epub 2019 May 6. PMID: 31170707
- Ballotta A, Lamti M, Kandil H, Varrica A, Bettini F, Satriano A, Isgrò G, Cotza M, Micheletti A, Ranucci M. ECMO in Combination with CytoSorb in a Woman with Para-Prosthetic Leak Following Mitral Valve Replacement, Candidate to Percutaneous Treatment: A Case Report Blood Purif. 2019; 47 Suppl 4:1. doi: 10.1159/000500178. Epub 2019 May 6. PMID: 31170707
- Manini E, Volpi F, Mencarelli F, Bocci F, Sini P, Ciampichini R, Todisco C, Beato V, Capini E. Hemoperfusion with CytoSorb for Bilirubin and Cytokine Removal in a Cardiac Surgery. Patient Blood Purif. 2019; 47 Suppl 4:1. doi: 10.1159/000500178. Epub 2019 May 6. PMID: 31170707
- Steurer LM, Schlager G, Sadeghi K, Golej J, Wiedemann D, Hermon M. Hemadsorption as rescue therapy for patients with multisystem organ failure in pediatric intensive care-Report of two cases reports and review of the literature. Artif Organs. 2021 Dec;45(12):1582-1593. doi: 10.1111/aor.14047. Epub 2021 Aug 12. PMID: 34331775; PMCID: PMC9291205.
- Scharf C, Liebchen U, Paal M, Becker-Pennrich A, Irlbeck M, Zoller M, Schroeder I. Successful elimination of bilirubin in critically ill patients with acute liver dysfunction using a cytokine adsorber and albumin dialysis: a pilot study. Sci Rep. 2021 May 13;11(1):10190. doi: 10.1038/s41598-021-89712-4. PMID: 33986443; PMCID: PMC8119427.
- Tomescu D, Popescu M, David C, Sima R, Dima S. Haemoadsorption by CytoSorb® in patients with acute liver failure: A case series. Int J Artif Organs. 2021 Aug;44(8):560-564. doi: 10.1177/0391398820981383. Epub 2020 Dec 10. PMID: 33302765.
- Acar U, Gökkaya Z, Akbulut A, Ferah O, Yenidünya Ö, Açık ME, Tokat Y, Yentür E. Impact of Cytokine Adsorption Treatment in Liver Failure. Transplant Proc. 2019 Sep;51(7):2420-2424. doi: 10.1016/j.transproceed.2019.01.167. Epub 2019 Aug 9. PMID: 31405742.
- Göth D, Mahler CF, Kälble F, Speer C, Benning L, Schmitt FCF, Dietrich M, Krautkrämer E, Zeier M, Merle U, Morath C, Fiedler MO, Weigand MA, Nusshag C. Liver-Support Therapies in Critical Illness-A Comparative Analysis of Procedural Characteristics and Safety. J Clin Med. 2023 Jul 13;12(14):4669. doi: 10.3390/jcm12144669. PMID: 37510784; PMCID: PMC10380554.
- Hui WF, Cheung WL, Hon KL, Ku SW. The application of hemoadsorption for hyperbilirubinemia and its impact on bilirubin removal kinetics in critically ill children. Int J Artif Organs. 2023 Apr;46(4):241-247. doi: 10.1177/03913988231163608. Epub 2023 Mar 24. PMID: 36964647.
- Popescu M, David C, Marcu A, Olita MR, Mihaila M, Tomescu D. Artificial Liver Support with CytoSorb and MARS in Liver Failure: A Retrospective Propensity Matched Analysis. J Clin Med. 2023 Mar 14;12(6):2258. doi: 10.3390/jcm12062258. PMID: 36983259; PMCID: PMC10058971.
- García-Villegas R, Arni S. Hemoadsorption in Organ Preservation and Transplantation: A Narrative Review. Life (Basel). 2023 Dec 29;14(1):65. doi: 10.3390/life14010065. PMID: 38255680; PMCID: PMC10817660.
- Ghinolfi D, Melandro F, Patrono D, Lai Q, De Carlis R, Camagni S, Gambella A, Ruberto F, De Simone P. A new ex-situ machine perfusion device. A preliminary evaluation using a model of donors after circulatory death pig livers. Artif Organs. 2022 Jul 4. doi: 10.1111/aor.14351. Epub ahead of print. PMID: 36136037.
- Usage and Application. CytoSorb.
- https://www.jafroninternational.com/therapiesproducts/products/bs_series
- Turan C, Szigetváry CE, Kói T, Engh MA, Atakan I, Zubek L, Terebessy T, Hegyi P, Molnár Z. Hemoadsorption Therapy for Critically Ill Patients with Acute Liver Dysfunction: A Meta-Analysis and Systematic Review. Biomedicines 2024; 12: 67. https://doi.org/10.3390/ biomedicines12010067
- Greimel A, Habler K, Gräfe C, Maciuga N, Brozat CI, Vogeser M, Zoller M, Happich FL, Liebchen U, Frank S, Paal M, Scharf C. Extracorporeal adsorption of protective and toxic bile acids and bilirubin in patients with cholestatic liver dysfunction: a prospective study. Ann Intensive Care. 2023 Nov 9;13(1):110. doi: 10.1186/s13613-023-01198-7. PMID: 37943350; PMCID: PMC10635921.
- Gräfe C, Paal M, Winkels M, Irlbeck M, Liebchen U, Scharf C. Correlation between Bilirubin Elimination with the Cytokine Adsorber CytoSorb® and Mortality in Critically Ill Patients with Hyperbilirubinemia. Blood Purif. 2023;52(11-12):849-856. doi: 10.1159/000532059. Epub 2023 Oct 11. PMID: 37820591.