More Information
Submitted: March 07, 2024 | Approved: March 15, 2024 | Published: March 18, 2024
How to cite this article: Stasilo R, Vinikovas A. Porphyria Cutanea Tarda (PCT) in a Patient, Treated with Hemodialysis after a Kidney Transplant Rejection Reaction: A Case Report. J Clini Nephrol. 2024; 8: 039-041.
DOI: 10.29328/journal.jcn.1001124
Copyright License: © 2024 Stasilo R, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: Porphyria cutanea tarda; Renal transplant rejection; Hemodialysis; Phlebotomy
Porphyria Cutanea Tarda (PCT) in a Patient, Treated with Hemodialysis after a Kidney Transplant Rejection Reaction: A Case Report
Radoslavas Stasilo1* and Artūras Vinikovas2
1Institute of Clinical Medicine, Faculty of Medicine, Vilnius University, Vilnius, Lithuania
2Gastroenterology, nephrology and surgery clinic of Faculty of Medicine, Vilnius University, Vilnius, Lithuania
*Address for Correspondence: Radoslavas Stasilo, Institute of Clinical Medicine, Faculty of Medicine, Vilnius University, Vilnius, Lithuania, Email: raddek365@gmail.com
Porphyrias are a group of inherited metabolic disorders of haem biosynthesis, involving a deficiency in the enzyme uroporphyrinogen decarboxylase. In this case report we present a case of a patient with porphyria cutanea tarda (PCT). A 40-year-old man on hemodialysis after a kidney transplant rejection reaction, complaining of skin changes, with a history of smoking and alcohol intake. Treated with Fusidic acid Betamethasone cream, and erythropoietin. Porhyria cutanea tarda can be considered in a patient who complains of skin changes. History of alcohol intake, smoking, high ferritin levels, and increased hepatic markers can raise suspicion of disease. In patients with ESRD treatment with erythropoietin, SPF 50+ sun cream, Fusidic acid, and Betamethasone can be effective.
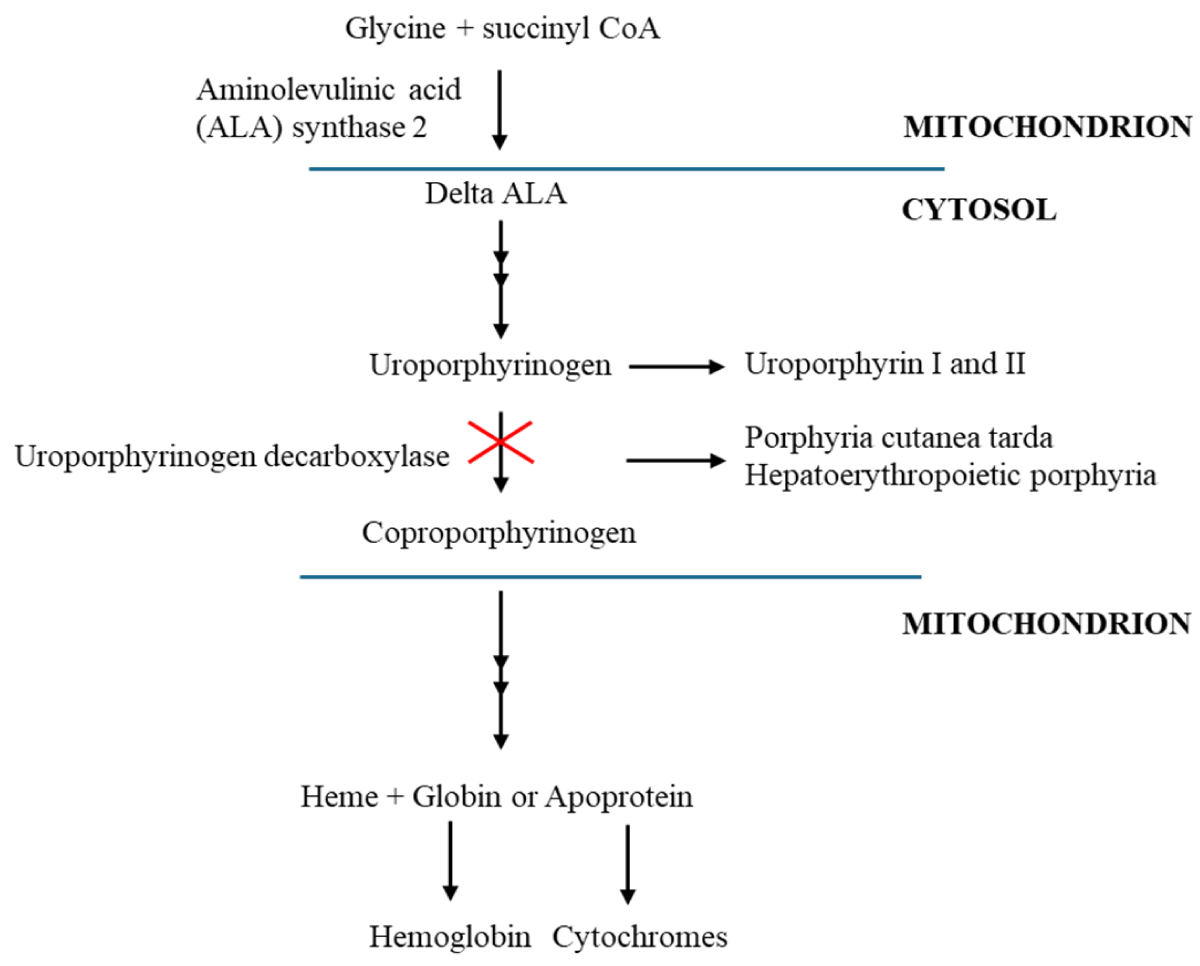
Porphyrias are a group of inherited metabolic disorders of haem biosynthesis involving a deficiency of one of the eight enzymes of the porphyrin–heme biosynthetic pathway. Each results from a specific enzymatic alteration in the haem biosynthesis pathway, which begins in the mitochondria and, after four cytoplasmic stages, ends at the same place (Figure 1) [1,2]. Porphyria Cutanea Tarda (PCT) is the most frequent type of porphyria worldwide, the prevalence of this disease ranges from 1 in 5000 to 25,000 individuals. Is more common in Czechoslovakia at 1 in 5000 in the general population, a 1 in 25000 prevalence has been reported in the United States [3,4]. PCT occurs in less than 5% of dialysis patients [5]. We describe a rare case of a patient with a kidney transplant rejection reaction who was treated with hemodialysis, had mildly elevated liver markers, and was diagnosed as having PCT which regressed without phlebotomy and chelating agents.
Figure 1: Haem biosynthetic pathway and pathogenesis of porphyria cutanea tarda with uroporphyrinogen decarboxylase mutation. Adapted from Muschalek, et al. [2].
A 40-year-old man presented, complaining of skin changes. Vesicles first time appeared in 2022 on arms after heat burn. Rashes regressed in winter and in the 2023 summer recurred, and vesicles were seen on the palms, neck, face, and leg. There was no family history of porphyria. Since 2004 the patient was diagnosed with chronic glomerulonephritis, a kidney biopsy was performed, and global and segmental glomerulosclerosis was found. Two years later, the patient underwent a living donor kidney transplant. In 2018, a biopsy confirmed acute rejection occurred. Unfortunately, despite steroid treatment, rituximab, and plasmapheresis, hemodialysis was started 3 times a week from 02/04/2020. In 2021 September the patient was added to the kidney transplant waitlist. He had hypertension and was cured of gastrointestinal bleeding. The patient smoked 20 cigarettes per day for 20 years and drank 10 standard drinks a week for 10 years. His medications included: Sol. Epoetini alfa, 4000 IU/0,4 ml 2 times weekly; 100 mg iron infusions every two weeks; tab. Nitrendipini, 20 mg daily; tab. Doxazosini, 4 mg daily; tab. Moxonidini 0,4 mg daily; tab. Bisoprololi 5 mg daily; tab. Perindoprili, 10 mg daily.
On admission patient’s respiratory rate was 16 times per minute SpO2 98%, blood pressure was 151/107 mm/Hg, and on legs, mild edema was seen. The patient had erythemic spots with crusting on the dorsal surface of the palm. Initial laboratory results revealed only high ferritin 1251, 3 qg/l (normal 20 – 500), elevated AST 177 U/L (normal 0 – 40), and ALT 62 U/L (normal 0 – 40), Haemoglobin (HGB) (g/l): 123 (normal 128 – 160), mean corpuscular volume (MCV) 102,6 fl (normal 78 – 96) Hepatitis markers, HIV test and autoimmune screening were negative. Any infectious diseases were not suspected. Abdominal ultrasound and esophagogastroduodenoscopy were performed. Only steatohepatitis was revealed.
Based on patient history and clinical evaluation, porphyria cutanea tarda disease was suspicious. Whole patient genome sequencing was performed. Investigation revealed that the patient had UROD gene pathogenetic heterozygous mutation which is typical for porphyria cutanea tarda and pathogenetic heterozygous mutation in INF 2 gene, which can lead to focal–segmental glomerulosclerosis.
Treatment with SPF 50+ sun cream, Fusidic acid, and Betamethasone cream daily was added to the previous treatment. Iron infusions were stopped. The patient was recommended to avoid sun exposure, stop drinking, and quit smoking. Previous medications were continued. Vesicles on the skin disappeared, and ferritin after six months was 328 qg/l.
PCT presents with skin symptoms only. Patients can present with increased skin fragility, subepidermal bullae, erosions, hypertrichosis, hyperpigmentation, hypo-pigmentation, milk spots, and scarring [1]. PCT can occur either in the absence or presence of an inherited UROD mutation. The heterozygous mutation causes half-normal levels of UROD activity systemically. This mutation can be mentioned as an etiological factor of PCT, but it alone is not enough, some other factors are needed to reduce the activity of UROD [6]. During End-Stage Renal Disease (ESRD), uraemic toxins, which accumulate in the body, iron overload, and hepatitis B and C affect the biosynthetic pathway of haem by reducing UROD activity (Figure 2) [7].
Figure 2: Role of ESRD in the development of PCT. Adapted from Pallet, et al. [7].
Although our presented patient’s hepatitis B and C markers were negative, iron overload was present and uremic toxins could have an impact on PCT because the patient had ESRD and was on hemodialysis. Also, alcohol and smoking, which were present in clinical cases, are often aggravating factors of PCT [8,9]. Alcohol intake increases iron absorption, stimulates free radical production, and is independently hepatotoxic. Elevated liver markers could be due to iron overload, which is hepatotoxic. Alcohol intake could also raise AST and ALT [10]. In rare cases, liver cirrhosis can be attributable to PCT but in our case, it was improbable [11]. Unlike other cutaneous porphyrias, there are effective treatments for PCT. Avoiding the sun, wearing protective clothing, and using SPF 50+ sunscreen can reduce skin symptoms. Phlebotomy is the treatment of choice in PCT. The main goal is iron depletion, usually achieved by 2‐weekly venesection of 250 to 500 mL of blood once to twice per week because of the associated anemia [12,13]. Treatment of PCT in hemodialysis patients is more complicated because they are typically anemic and do not tolerate phlebotomy. Antimalarial is also ineffective because of the impaired urinary excretion of chloroquine-porphyrin complexes [14]. Erythropoietin mobilizes hepatic iron for hemoglobin synthesis and is the treatment of choice for PCT in renal failure, usually used in doses ranging from 20 to 50 U/kg 2 to 3 times a week [12,13]. Treatment with deferoxamine or deferasirox can be efficient but can cause a ferropenic state and is less efficient than phlebotomy [5,15]. The patient, in this case, continued to take erythropoietin, iron infusions were stopped and local treatment was started. Initial treatment was effective.
Clinicians should consider porhyria cutanea tarda in every hemodialysis patient who complains of skin changes. History of alcohol intake, smoking, high ferritin levels, and increased hepatic markers can raise suspicion of this rare disease. In patients with ESRD treatment with erythropoietin, SPF 50+ sun cream, Fusidic acid, and Betamethasone can be effective.
Ethical approval
Written informed consent was obtained from the patient for publication of this case report. A copy of the written consent is available for review by the editor–in–chief of this journal on request.
- Puy H, Gouya L, Deybach JC. Porphyrias. Lancet. 2010 Mar 13;375(9718):924-37. doi: 10.1016/S0140-6736(09)61925-5. PMID: 20226990.
- Muschalek W, Hermasch MA, Poblete-Gutiérrez P, Frank J. The Porphyrias. J Dtsch Dermatol Ges. 2022 Mar;20(3):316-331. doi: 10.1111/ddg.14743. PMID: 35304965.
- Chan OT, Tsai N, Wong RL, Izumi AK. The additive effects of hepatitis C infection and end-stage renal disease in porphyria cutanea tarda. Cutis. 2006 Dec;78(6):397-400. PMID: 17243426.
- Singal AK. Porphyria cutanea tarda: Recent update. Mol Genet Metab. 2019 Nov;128(3):271-281. doi: 10.1016/j.ymgme.2019.01.004. Epub 2019 Jan 18. PMID: 30683557.
- Rodrigues N, Caeiro F, Santana A, Mendes T, Lopes L. Porphyria Cutanea Tarda in a Patient with End-Stage Renal Disease: A Case of Successful Treatment with Deferoxamine and Ferric Carboxymaltose. Case Rep Nephrol. 2017; 2017:4591871. doi: 10.1155/2017/4591871. Epub 2017 Jan 22. PMID: 28210512; PMCID: PMC5292176.
- Weiss Y, Chen B, Yasuda M, Nazarenko I, Anderson KE, Desnick RJ. Porphyria cutanea tarda and hepatoerythropoietic porphyria: Identification of 19 novel uroporphyrinogen III decarboxylase mutations. Mol Genet Metab. 2019 Nov;128(3):363-366. doi: 10.1016/j.ymgme.2018.11.013. Epub 2018 Nov 28. PMID: 30514647; PMCID: PMC8132452.
- Pallet N, Karras A, Thervet E, Gouya L, Karim Z, Puy H. Porphyria and kidney diseases. Clin Kidney J. 2018 Apr;11(2):191-197. doi: 10.1093/ckj/sfx146. Epub 2018 Jan 10. PMID: 29644058; PMCID: PMC5888040.
- Tang Z, Ma J, Wang L, Shi Z. Porphyria cutanea tarda associated with alcohol abuse: three case reports. Preprints; 2022 Jul [cited 2024 Mar 14]. https://www.authorea.com/users/494199/articles/576343-porphyria-cutanea-tarda-associated-with-alcohol-abuse-three-case-reports?commit=989ce8f8e16e3c5ead989839f62a862c2a72e288
- Egger NG, Goeger DE, Payne DA, Miskovsky EP, Weinman SA, Anderson KE. Porphyria cutanea tarda: multiplicity of risk factors including HFE mutations, hepatitis C, and inherited uroporphyrinogen decarboxylase deficiency. Dig Dis Sci. 2002 Feb;47(2):419-26. doi: 10.1023/a:1013746828074. PMID: 11855561.
- Usta Atmaca H, Akbas F. Porphyria cutanea tarda: a case report. J Med Case Rep. 2019 Jan 21;13(1):17. doi: 10.1186/s13256-018-1956-9. PMID: 30661508; PMCID: PMC6340172.
- Lee KG, Hyun JJ, Seo YS, Keum B, Yim HJ, Jeen YT, Lee HS, Chun HJ, Kim CD, Ryu HS, Um SH. Liver cirrhosis induced by porphyria cutanea tarda: a case report and review. Gut Liver. 2010 Dec;4(4):551-5. doi: 10.5009/gnl.2010.4.4.551. Epub 2010 Dec 17. PMID: 21253308; PMCID: PMC3021615.
- Sarkany RP. The management of porphyria cutanea tarda. Clin Exp Dermatol. 2001 May;26(3):225-32. doi: 10.1046/j.1365-2230.2001.00825.x. PMID: 11422163.
- Shieh S, Cohen JL, Lim HW. Management of porphyria cutanea tarda in the setting of chronic renal failure: a case report and review. J Am Acad Dermatol. 2000 Apr;42(4):645-52. PMID: 10727312.
- Huang YC, Wang CC, Sue YM. Porphyria cutanea tarda in a hemodialysis patient. QJM. 2013 Jun;106(6):591-2. doi: 10.1093/qjmed/hcs115. Epub 2012 Jun 28. PMID: 22753666.
- Bissell DM, Anderson KE, Bonkovsky HL. Porphyria. N Engl J Med. 2017 Aug 31;377(9):862-872. doi: 10.1056/NEJMra1608634. PMID: 28854095.