More Information
Submitted: April 29, 2024 | Approved: May 10, 2024 | Published: May 13, 2024
How to cite this article: Sánchez MB, Barrero S, Da Silva MR, Martinez C, Tirado GM, et al. Elimination of Medium/High Molecular Weight Solutes. Comparison of High Flow Hemodialysis with Extended Hemodialysis. J Clini Nephrol. 2024; 8: 066-075.
DOI: 10.29328/journal.jcn.1001129
Copyright License: © 2024 Sánchez MB, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: Extended hemodialysis; Depurative efficacy; Reduction ratio; β2 Microglobulin; IL-6; Inflammation; Calprotectin
Elimination of Medium/High Molecular Weight Solutes. Comparison of High Flow Hemodialysis with Extended Hemodialysis
Manuel Benítez Sánchez*, Sergio Barrero, Maria R Da Silva, Carlos Martinez, Guillermo Manuel Tirado and Sonia Cruz
Nephrology Service, Hospital Universitario Juan Ramón Jiménez, Spian
*Address for Correspondence: Manuel Benítez Sánchez, Nephrology Service, Juan Ramón Jiménez Hospital, North Round s/n. 21003, Huelva, Spain, Email: [email protected]
Introduction: Post-dilution online hemodiafiltration is the most efficient extracorporeal depurative treatment of CKD. Recently a new type of membrane has been developed, with a higher cut-off point also called a medium cut-off point, which has the capacity to eliminate higher molecular weight molecules in hemodialysis. The hemodialysis performed with these membranes has been called “Expanded Hemodialysis”.
Objective: Compare the purifying efficacy of medium and high molecular weight molecules in patients dialyzed with high-flux hemodialysis, VitaPES 210HF, and with patients treated with expanded hemodialysis with the medium cut-off dialyzer, Elisio-HX.
We also assessed the effect that the increased removal of inflammatory mediators by MCO hemodialysis had on fecal Calprotectin levels.
Patients and methods: This is a prospective observational cross-over study in which 8 prevalent hemodialysis patients were followed for two months. Blood levels of IL-6, C-reactive protein (CRP), β2-microglobulin, Kappa and Lambda immunoglobulin light chains, and serum albumin were determined before and after each hemodialysis session.
Results: The percentage of reduction of medium and higher molecular weight molecules: β2microglobulin, IL-6, Kappa and Lambda chains and CRP were higher with the Elisio-21HX dialyzer compared to the VitaPES 210HF dialyzer. There was no difference in albumin clearance between the two dialyzers.
Fecal calprotectin levels were lower in patients dialyzed with Elisio-21HX.
Conclusion: The medium cutoff dialyzer, Elisio-HX, is more efficient in the elimination of medium/high molecular weight molecules than the VitaPES HF high-flux dialyzer, with the same albumin elimination.
Improving inflammation at the local intestinal level with lower levels of fecal Calprotectin.
The use of high-flux hemodialysis (HD-HF) and online hemodiafiltration (OL-HDF) has required advances in hemodialysis technique. Among them, are the use of machines with precise control of Ultrafiltration (UF), dialysis fluids with bicarbonate, and ultrapure water quality. Expanded Hemodialysis (HDx) requires a dialysis monitor with these advances, although, unlike OL-HDF, it does not require a dialysate infusion system linked to UF control. HDx can be performed with any modern HD machine with an endotoxin filter.
HDx with Medium Cut-Off membranes (MCO), designed to improve the permeability of dialysis membranes, has been incorporated into clinical practice, and although these dialyzers can only be used in the hemodialysis modality, they could provide an alternative to OL-HDF, since they achieve in certain cases the same elimination performance as OL-HDF in post-dilution [1]. Expanded hemodialysis, extended hemodialysis, and hemodialysis with a medium-cutoff dialyzer are synonymous and used interchangeably.
This is related to the specific pore cut-off size, combined with an internal architecture that allows the MCO membranes to optimize internal convection and thus increase the clearance capacity of medium and large molecules relative to standard high-flux HD treatments. While maintaining high solute clearance of less than 10 kDa, these MCO membranes were developed to improve the clearance of medium to high molecular weight (MW) solutes (in the range of 10 to 50 kDa) [2].
CKD is associated with local and systemic inflammation, and evidence of systemic inflammation has been associated with worse survival. The gastrointestinal tract is an important source of chronic inflammation in CKD, both because of uremic toxins released by the microbiota, and because of increased intestinal permeability that allows access of bacterial products to the circulation. Bacterial DNA derived from the intestinal circulation was detected in patients with CKD and correlated with increased plasma C-reactive protein (CRP) and interleukin-6 (IL-6), in the absence of infection.
Also known as the “second human genome,” the gut microbiome plays an important role in both the maintenance of health and the pathogenesis of disease.
The symbiotic relationship between the host and the microbiome is altered due to the proliferation of dysbiotic bacteria in patients with Chronic Kidney Disease (CKD). Fermentation of proteins and amino acids by intestinal bacteria generates excessive amounts of potentially toxic compounds, such as ammonia, amines, thiols, phenols, and indoles, but the generation of healthy products such as short-chain fatty acids is reduced. Altered intestinal barrier function in CKD allows the translocation of gut-derived uremic toxins into the systemic circulation, contributing to CKD progression, uremic toxicity, systemic inflammation, cardiovascular disease, insulin resistance, and protein energy loss [3].
Fecal calprotectin levels correlated positively with age and metabolic phenotypes (Body Mass Index (BMI), diabetes, statin, and metformin use, Hg A1c, and systolic blood pressure), but correlated negatively with consumption of vegetables, vegetable proteins, chocolate, and bread, it has recently been published that the determination of fecal chromogranin and calprotectin correlate well with fruit and vegetable intake and with the richness and diversity of the microbiota.
Thus, high fecal chromogranin and high fecal calprotectin correlate well with low gut microbiota diversity in metagenomic studies and with high intestinal permeability which is a well-documented feature in CKD patients [4].
Fecal calprotectin is a biomarker of intestinal inflammation often used to monitor the activity of patients with inflammatory bowel disease and their response.
Patients treated with proton pump inhibitors are known to spuriously alter fecal calprotectin levels.
The objective of our study was to evaluate the safety and efficacy of this new MCO Elisio21HX dialyzer and to compare it with the VitaPES 210HF high-flux membrane hemodialysis treatment.
A secondary objective of our study was to determine how the increased elimination of inflammatory cytokines such as IL-6, and CRP by Extended Hemodialysis translated into improvement of local intestinal inflammation for which we determined Fecal Calprotectin.
Efficiency in the clearance of medium/higher molecular weight molecules with the Elisio-21HX dialyzer is higher than with the VitaPES 210HF dialyzer.
The elimination of these molecules is related to the improvement of inflammatory parameters.
General and specific objectives
Overall objective: The objective is to study different biomarkers of inflammation in hemodialysis patients, comparing the clearance efficacy of high-flux hemodialysis in patients dialyzed with VitaPES 210HF with the clearance efficacy of extended hemodialysis in patients dialyzed with the super high flux or medium cut-off Elisio-21HX dialyzer. The characteristics of both dialyzers appear in (Figure 1).
Figure 1: Dialyzer Characteristics.
Specific objectives: As a secondary objective, the effect of this inflammatory medium and its possible change on a marker of the intestinal microbiota profile and intestinal inflammation, such as fecal calprotectin, was evaluated, comparing it with the different dialyzers.
Design
This was a prospective observational study.
Population and sample
Inclusion criteria
Stable patients on hemodialysis who are over 18 years of age and give informed consent to participate in the study, Likewise, the study protocol was approved by the ethical committee of the Andalusian Health Service, managed by the entity promoting the study, the FABIS foundation, Fundación Andaluza BETURIA for Health Research with the protocol number: FAB-ELI-2022-01
There are 8 prevalent patients on hemodialysis, 4 men and 4 women, the mean age was 68 years. They were at the time of the beginning of the study being dialyzed with the Vitapes 210 dialyzer. The hemodialysis regimen was 4 hours of duration 3 times per week, vascular access was a central venous catheter in 6 of the 8 patients, and the dialysis bath was customized for each patient as is standard practice in the unit.
All patients were first dialyzed with the VitaPES 210HF dialyzer for one month after a 1 week washout period, during which the dialyzer used was different from the two filters studied, we used a Polymethyl Methacrylate membrane during this week, the same 8 patients were dialyzed with the Elisio-21HX dialyzer with a 1-month follow-up. The dialysis parameters collected in each session were:
Actual session duration, arterial pressure, venous pressure, initial and final body weight, and volume of blood processed. UF ml/kg/h rate, refilling rate (Figure 2).
Figure 2: Homodialysis Parameters.
After approval of our study protocol by the Hospital Ethics Committee, the fieldwork was carried out between May-July / 2023. Data analysis started in September 2023.
Exclusion criteria
Exclusion criteria are malnutrition states and patients undergoing Hemicolectomy. Patients diagnosed with Chronic Inflammatory Bowel Disease were also excluded from the study. Also excluded from the study were those patients who were being treated with proton pump inhibitors, which are known to spuriously alter fecal Calprotectin levels.
Variables
Blood concentrations of IL-6 (26,000 Da) were determined before and after the hemodialysis session and at three points in time: at the beginning, middle, and end of the month of treatment with each dialyzer, and measured in pg/ml, CRP (135.000 Da) and measured in mg/L, as markers of inflammatory cytokines and high Molecular Weight, β2- Microglobulin (MW 11,800 Da) in mg/L, as medium MW molecule and Kappa light chains (MW22,500 Da) and Lambda (45,000 Da) both in mg/L. All patients had their serum albumin level (MW 66,000 Da) measured before and after each hemodialysis session (Figure 3). shows the minimum value, first quartile, median, mean, third quartile, and maximum value of each of the pre-and post-dialysis concentrations of all the variables studied and dialyzed with Elisio-21HX, and shows the same data for patients dialyzed with VitaPES 210HF.
Figure 3: Molecular Concentration. C1-Pre and C2-Post dialysis.
Fecal Calprotectin concentration mg/kg was determined in all patients at the beginning and end of the treatment period with each dialyzer.
There is no main variable in the study but a set of analytical determinations of different medium and high molecular weight molecules. For the secondary objective, the target variable will be the fecal Calprotectin level.
Source of information and data
The efficacy of hemodialysis in the elimination of small molecules was measured according to Kt/v, Kt referred to the body surface area of each patient. To determine the clearance of medium and large molecules, the ratio between the post-dialysis concentration (C2) and the pre-dialysis concentration (C1) of each molecule studied was measured, calculating the Reduction Ratio (RR). RR = C2/C1. The percentage of reduction of each molecule in the hemodialysis session was obtained with the following formula: % Reduction = 1- (RR) X 100.
Data analysis
Using R-Studio statistical software, a descriptive analysis of both sociodemographic and clinical variables was performed. Tests were performed to determine the adjustment to normality of the quantitative variables, in order to determine the use of parametric or nonparametric statistics. Adjustment to normality was performed using the Shapiro-Wilk test. The t-test was used for the comparison of means. In all cases, a statistical significance of 5% (p < 0.05) was required.
As it is a synthetic membrane, hypersensitivity reactions are possible.
Adequate tolerance to the two dialyzers was observed, with no adverse reactions during connection and disconnection or during hemodialysis sessions.
In terms of depurative efficacy, we will examine each molecule separately (Figure 4).
Figure 4: Reduction Ratio-RR. Comparison of means-t-test.
Albumin
The %Albumin RR with VitaPES HF was 4% and the %Albumin RR with Elisio-HX was 8%. There is no significant difference in albumin RR between VitaPES and Elisio-HX t-test two-sided p - value = 0.27 Confidence interval two-sided p = -0.0308, 0.1182. The difference in albumin removal with both dialyzers was not statistically significant.
Medium molecular weight molecules: Up to 15.000 Da of MW
Beta 2 Microglobulin (PM 11,800 Da) % Beta2 RR with VitaPES was 65.2% and with ElisioHX the % Beta2 RR was 71.4%.
We found statistically significant differences when comparing the elimination of β2Microglobulin being higher with the Elisio-HX dialyzer, with a two-sided p - value p = 0.0044, and the confidence interval p two-sided: 0.0204, 0.1046.
High molecular weight molecules between 15,000 and 60,000 Daltons
Interleukin 6, IL-6 (Molecular weight 26,000 Da): The %IL-6 RR was 17% with the VitaPES HF dialyzer and 25% with the Elisio-HX dialyzer. We found statistically significant differences p two-sided p = 0.038 Confidence interval p two-sided: 0.004, 0.1471.
Thus, IL-6 clearance was statistically significantly higher with the Elisio-HX dialyzer compared to VitaPES HF.
C-reactive protein -PCR (molecular weight 135,000 Da): The %PCR- RR with the VitaPES HF dialyzer was 7.46% and 11.37% with Elisio-HX.
No significant differences were found p two-sided, p = 0.0817. The confidence interval p two-sided: -0.0833, 0.0051.
Immunoglobulin light chains
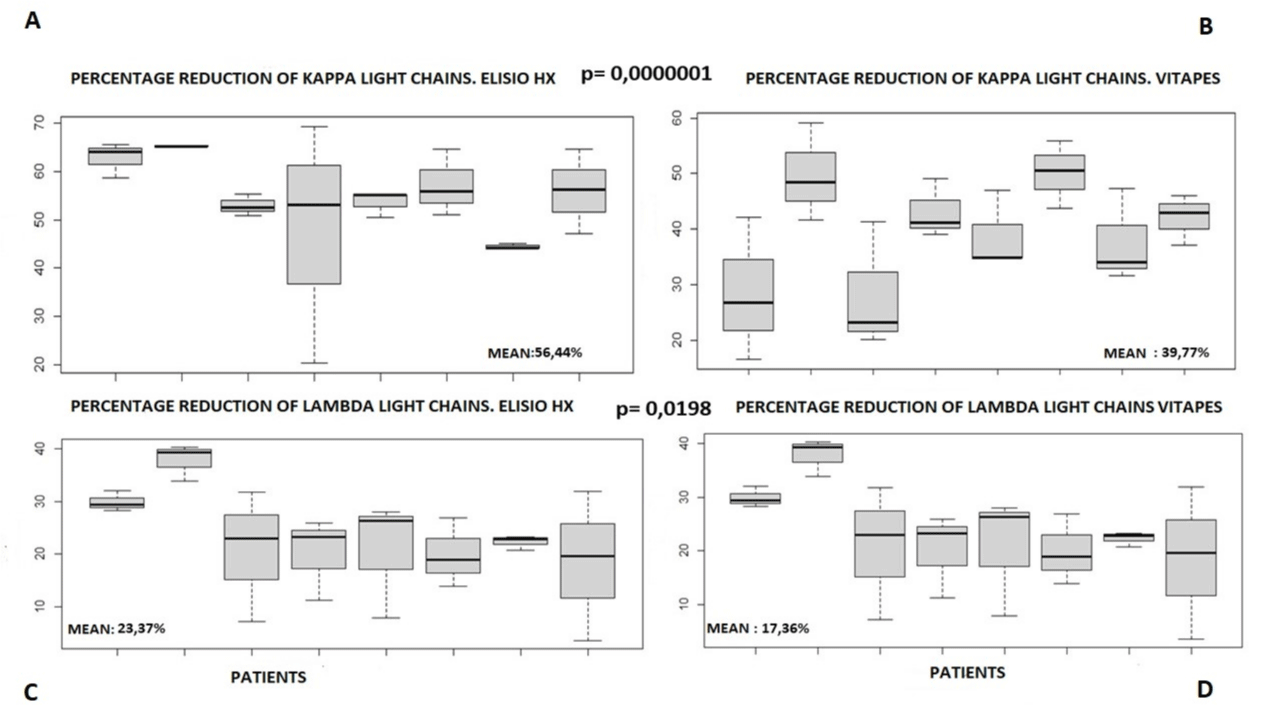
Kappa chain (molecular weight 22,500 Da): The % kappa RR with VitaPES HF was 39.77% compared to 56.44% with Elisio-HX.
The mean clearance of Kappa light chains with the Elisio-21HX dialyzer was 56.44% which is statistically significantly higher than the clearance of Kappa chains with the VitaPES 210HF dialyzer which was 39.77%.
p two-sided = 0.0000001, and the confidence interval: 0.1149, 0.2185
Lambda chains (molecular weight 45,000 Da): The mean Lambda light chain removal with Elisio-HX was 23.37% which was higher than with VitaPES HF which was 17.36%, statistically significantly, p: 0.01984. The confidence Interval p two-sided: 0.0099, 0.1103.
Box-and-whisker plots of the mean comparisons for each of the molecules studied, individually for each patient, are shown in Figures 5-7.
Figure 5: Box-and-whisker plot. Mean comparisons. Individual patient.
Figure 6: Box-and-whisker plot. Mean comparisons. Individual patient.
Figure 7: Box-and-whisker plot. Mean comparisons. Individual patient.
Fecal analysis
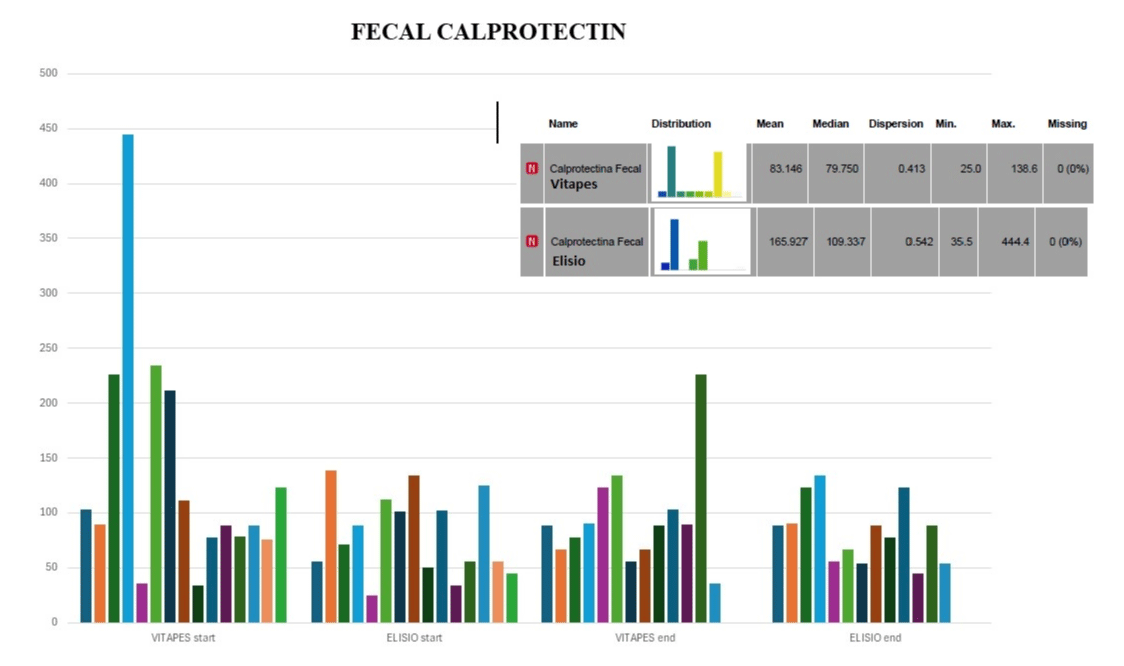
Figure 8 shows the fecal calprotectin concentrations at the beginning and end of the treatment period with each dialyzer. There were no statistically significant differences with the t-test comparing means between calprotectin concentrations at the beginning and at the end of treatment with each of the dialyzers.
Figure 8: Fecal Calprotectin.
In the group of patients dialyzed with Elisio-21HX, a predictive model of fecal calprotectin concentration was performed by multivariate linear regression. An inverse relationship was found between fecal calprotectin concentration and RR-IL-6 rate, RR-CRP rate, and post-hemodialysis IL-6 and CRP concentrations. And a direct relationship between fecal Calprotectin and predialysis blood levels of CRP and IL-6, in a statistically significant manner (Figure 9).
Figure 9: Elisio HX. Calprotectin. Multivariate Linear Regression.
The results of the multivariate linear regression performed in the group of patients dialyzed with VitaPES 210HF did not yield results with statistical significance, data not shown.
In our study we have found that the Hx dialyzer decreases tissue inflammation at the intestinal level by increasing the RR % of CRP and IL-6 and decreasing the predialysis blood concentration of CRP and IL-6, which are inversely and directly related respectively to the level of fecal calprotectin, the biomarker par excellence of intestinal inflammation.
Our results show that blood concentrations of Beta 2 microglobulin, IL-6, Kappa light chains, Lambda light chains, and CRP are reduced to a greater extent with the MCO Elisio-HX dialyzer compared to the VitaPES High-flux dialyzer. This underlines the ability to reduce the inflammatory level in chronic dialysis patients obtained with the Elisio-HX dialyzer and what this entails in terms of improving morbidity and mortality and the quality of life of these patients. We found no differences in albumin elimination with both dialyzers. What does it entail?
It implies that the Elisio-Hx dialyzer improves systemic and local inflammation due to its greater removal of inflammatory cytokines with low albumin loss, which is beneficial and is the same removal as with high-flow hemodialysis.
There are 16 ongoing HDx studies registered in ClinicalTrials.gov. Six of these are complete and eight are under recruitment. Six compare HDx with HD-High Flux, four with HDF, and one with both techniques. Some focus on specific aspects such as anticoagulation, preservation of RRF, calcifications, and mineral metabolism. Among them is the MoTHER study, a Spanish multicenter, open, prospective, randomized, prospective study to explore morbidity and mortality in patients dialyzed with HDx compared to OL-HDF, its objective is to determine whether HDx is non-inferior to OL-HDF in reducing the combined outcome of all-cause death and stroke (ischemic or hemorrhagic) and acute coronary syndrome (angina and myocardial infarction) and peripheral arterial disease event (amputation or revascularization) and ischemic colitis (mesenteric thrombosis) in subjects on HD [5].
With MCO dialyzers, albumin loss in dialysis fluid is usually greater than in HD-High Flux and in OL-HDF, in any case, less than 3.5 g/session, between 0.03 and 3.15 g/session. Albumin loss depends on the type of membrane and the transmembrane pressures used and can exceed 10 g/4 h in OL-HDF with some dialyzers [6]. In patients on HDx, serum albumin is maintained after an initial drop [7]. At 12 weeks and 12 months with MCO membranes, no significant changes in albuminemia have been detected [8]. In the work of Bunch, et al. [9] performed on 638 patients, after one year, they found a decrease of 3.5%.
There is little evidence on medium and long-term clinical results with HDx, the study that includes more patients is the Colombian COREXH Registry [7], where 992 patients dialyzed with HDx were recruited and 638 completed one year of follow-up. They have a mortality of 8.54 per 100 patient-years, which is low compared to other similar studies with other hemodialysis techniques [10]. In our study, we found no differences in albumin clearance in patients on high-flux hemodialysis dialyzed with VitaPES-210HF and patients treated with extended hemodialysis dialyzed with Elisio-21HX.
To HDx has been reported an improvement in the parameters.
Proinflammatory [11]. One of the factors that explain the loss of Residual Renal Function (RRF) in dialysis is inflammation [12]. Some high PM TU would be detrimental to renal tubules and to RRF [13], their increased clearance by MCO dialyzers [14] could better preserve RRF, which will have to be investigated in the future.
Another group of patients that could benefit from extended hemodialysis with Elisio-HX are patients diagnosed with Multiple Myeloma, especially if it is based on Kappa light chains.
In general, patients in a hemodialysis program who are not candidates for renal transplantation and who are expected to remain in a renal replacement program for a long time, patients with chronic inflammation in hemodialysis such as those with Erythropoietin Resistance Syndrome, intractable pruritus, vascular calcification, restless legs syndrome could be other potential candidates for hemodialysis with Elisio-HX dialyzer, would be eligible for treatment with extended hemodialysis with Elisio-HX.
As a strong point of our work, we can mention that the purifying efficacy of CRP measured as percentage reduction (%RR) (Table 1), which we have determined and which has been found to be higher in patients dialyzed with Elisio-HX, has not yet been reported in the literature.
| Table 1: Percentage Reduction During Hemodialysis. % Reduction: 1 – RR x 100. | |||||
| Vitapes RR |
Vitapes % Reduction |
Elisio HX RR | Elisio HX % Reduction |
Significance | |
| Albumin | 0.9593 | 4% | 0.9156 | 8% | p = 0.2403 |
| Interleukin 6 | 0.8233 | 17% | 0.7478 | 25% | p = 0.0388 |
| C-reactive protein | 0.8863 | 7,46% | 0.9254 | 11,37% | p = 0,0817 |
| Beta 2 microglobulin | 0.3481 | 65,2% | 0.2856 | 71,4% | p = 0.0044 |
| Light chains kappa | 0.6023 | 39,77% | 0.4356 | 56.44% | p = 0,0000001 |
| Light chains lamda | 0.8264 | 17.36% | 0.7663 | 23,37% | 0,01984 |
The increased elimination of inflammatory mediators that are achieved through extended hemodialysis improves the general inflammatory environment and after the results obtained in our study we can also say a decrease in inflammation at the intestinal level with the benefits that can be derived from this such as improvement of the increased intestinal permeability and increased synthesis of short-chain fatty acids, but these points require future studies that we will carry out in the near future.
Limitations of our study
The time interval to observe changes in calprotectin biomarkers and the clinical evolution of the patients studied, who were monitored for only two months, was too short; more follow-up time and the inclusion of intestinal microbiota variables would be necessary for future studies.
Thanks: To all the health personnel (nursing, clinical assistant, guards) of the Hemodialysis unit of the Juan Ramon Jimenez Hospital (Huelva), without whose work and effort, this work would not have been possible.
- Maduell F, Broseta JJ, Rodríguez-Espinosa D, Del Risco J, Rodas LM, Arias-Guillén M, Vera M, Fontseré N, Salgado MDC, Rico N. Comparison of four medium cut-off dialyzers. Clin Kidney J. 2022 Jul 13;15(12):2292-2299. doi: 10.1093/ckj/sfac167. PMID: 36381368; PMCID: PMC9664569.
- Boschetti-de-Fierro A, Voigt M, Storr M, Krause B. Extended characterization of a new class of membranes for blood purification: the high cut-off membranes. Int J Artif Organs. 2013 Jul;36(7):455-63. doi: 10.5301/ijao.5000220. Epub 2013 May 10. PMID: 23661558.
- Castillo-Rodriguez E, Fernandez-Prado R, Esteras R, Perez-Gomez MV, Gracia-Iguacel C, Fernandez-Fernandez B, Kanbay M, Tejedor A, Lazaro A, Ruiz-Ortega M, Gonzalez-Parra E, Sanz AB, Ortiz A, Sanchez-Niño MD. Impact of Altered Intestinal Microbiota on Chronic Kidney Disease Progression. Toxins (Basel). 2018 Jul 19;10(7):300. doi: 10.3390/toxins10070300. PMID: 30029499; PMCID: PMC6070989.
- Zhernakova A, Kurilshikov A, Bonder MJ, Tigchelaar EF, Schirmer M, Vatanen T, Mujagic Z, Vila AV, Falony G, Vieira-Silva S, Wang J, Imhann F, Brandsma E, Jankipersadsing SA, Joossens M, Cenit MC, Deelen P, Swertz MA; LifeLines cohort study; Weersma RK, Feskens EJ, Netea MG, Gevers D, Jonkers D, Franke L, Aulchenko YS, Huttenhower C, Raes J, Hofker MH, Xavier RJ, Wijmenga C, Fu J. Population-based metagenomics analysis reveals markers for gut microbiome composition and diversity. Science. 2016 Apr 29;352(6285):565-9. doi: 10.1126/science.aad3369. Epub 2016 Apr 28. PMID: 27126040; PMCID: PMC5240844.
- Ortiz PDS, Garcia RP, Vega A, Maduell F. Preliminary Data from MOTHER HDX Study: A Multicentre Open-label RCT Study to Explore the Morbimortality with the Theranova HDX vs OL-HDF. Nephrol Dial Transplant. 2023 Jun 1;38(Supplement_1):gfad063c_3472.
- Kirsch AH, Lyko R, Nilsson LG, Beck W, Amdahl M, Lechner P, Schneider A, Wanner C, Rosenkranz AR, Krieter DH. Performance of hemodialysis with novel medium cut-off dialyzers. Nephrol Dial Transplant. 2017 Jan 1;32(1):165-172. doi: 10.1093/ndt/gfw310. Erratum in: Nephrol Dial Transplant. 2021 Jul 23;36(8):1555-1556. PMID: 27587605; PMCID: PMC5837492.
- Boschetti-de-Fierro A, Beck W, Hildwein H, Krause B, Storr M, Zweigart C. Membrane Innovation in Dialysis. In: Ronco C, editor. Expanded Hemodialysis: Innovative Clinical Approach in Dialysis [Internet]. S.Karger AG; 2017 [cited Nov 28, 2023]. p. 0. Available from: https://doi.org/10.1159/000479259.
- Lim JH, Park Y, Yook JM, Choi SY, Jung HY, Choi JY, Park SH, Kim CD, Kim YL, Cho JH. Randomized controlled trial of medium cut-off versus high-flux dialyzers on quality of life outcomes in maintenance hemodialysis patients. Sci Rep. 2020 May 8;10(1):7780. doi: 10.1038/s41598-020-64622-z. PMID: 32385307; PMCID: PMC7210312.
- Bunch A, Sanchez R, Nilsson LG, Bernardo AA, Vesga JI, Ardila F, Guerrero IM, Sanabria RM, Rivera AS; Colombian Registry of Expanded Hemodialysis investigators. Medium cut-off dialyzers in a large population of hemodialysis patients in Colombia: COREXH registry. Ther Apher Dial. 2021 Feb;25(1):33-43. doi: 10.1111/1744-9987.13506. Epub 2020 May 29. PMID: 32352233; PMCID: PMC7818220.
- Maduell F, Varas J, Ramos R, Martin-Malo A, Pérez-Garcia R, Berdud I, Moreso F, Canaud B, Stuard S, Gauly A, Aljama P, Merello JI. Hemodiafiltration Reduces All-Cause and Cardiovascular Mortality in Incident Hemodialysis Patients: A Propensity-Matched Cohort Study. Am J Nephrol. 2017;46(4):288-297. doi: 10.1159/000481669. Epub 2017 Oct 17. PMID: 29041011.
- Zickler D, Schindler R, Willy K, Martus P, Pawlak M, Storr M, et al. Medium Cut-Off (MCO) membranes reduce inflammation in chronic dialysis patients - A randomized controlled clinical trial. PLoS ONE [Internet]. 2017;12(1). Available from: https://www.scopus.com/inward/record.uri?eid=2-s2.0-85009286665&doi=10.1371%2fjournal.pone.0169024&partnerID=40&md5=d17c993a178ed064067d53be07b0a560.
- de Sequera P, Corchete E, Bohorquez L, Albalate M, Perez-Garcia R, Alique M, Marques M, García-Menéndez E, Portolés J, Ramirez R. Residual Renal Function in Hemodialysis and Inflammation. Ther Apher Dial. 2017 Dec;21(6):592-598. doi: 10.1111/1744-9987.12576. Epub 2017 Oct 3. PMID: 28971592.
- Wolley MJ, Hutchison CA. Large uremic toxins: an unsolved problem in end-stage kidney disease. Nephrol Dial Transplant. 2018 Oct 1;33(suppl_3):iii6-iii11. doi: 10.1093/ndt/gfy179. PMID: 30281131; PMCID: PMC6168891.
- Latosinska A, Hulko M, Speidel R, Mischak H, Storr M, Krause B. Removal of Cell-Activating Substances Using Dialyzers With Various Permeability Profiles. Artif Organs. 2018 Jan;42(1):78-87. doi: 10.1111/aor.12952. Epub 2017 Jul 26. PMID: 28744941.