Figure 3
An update in the utilization of N-acetyl cysteine & vitamin c for tackling the oxidative stress in acute kidney injury secondary to robust sepsis - A systematic review
Kulvinder Kochar Kaur*, Gautam Allahbadia and Mandeep Singh
Published: 01 February, 2022 | Volume 6 - Issue 1 | Pages: 001-018
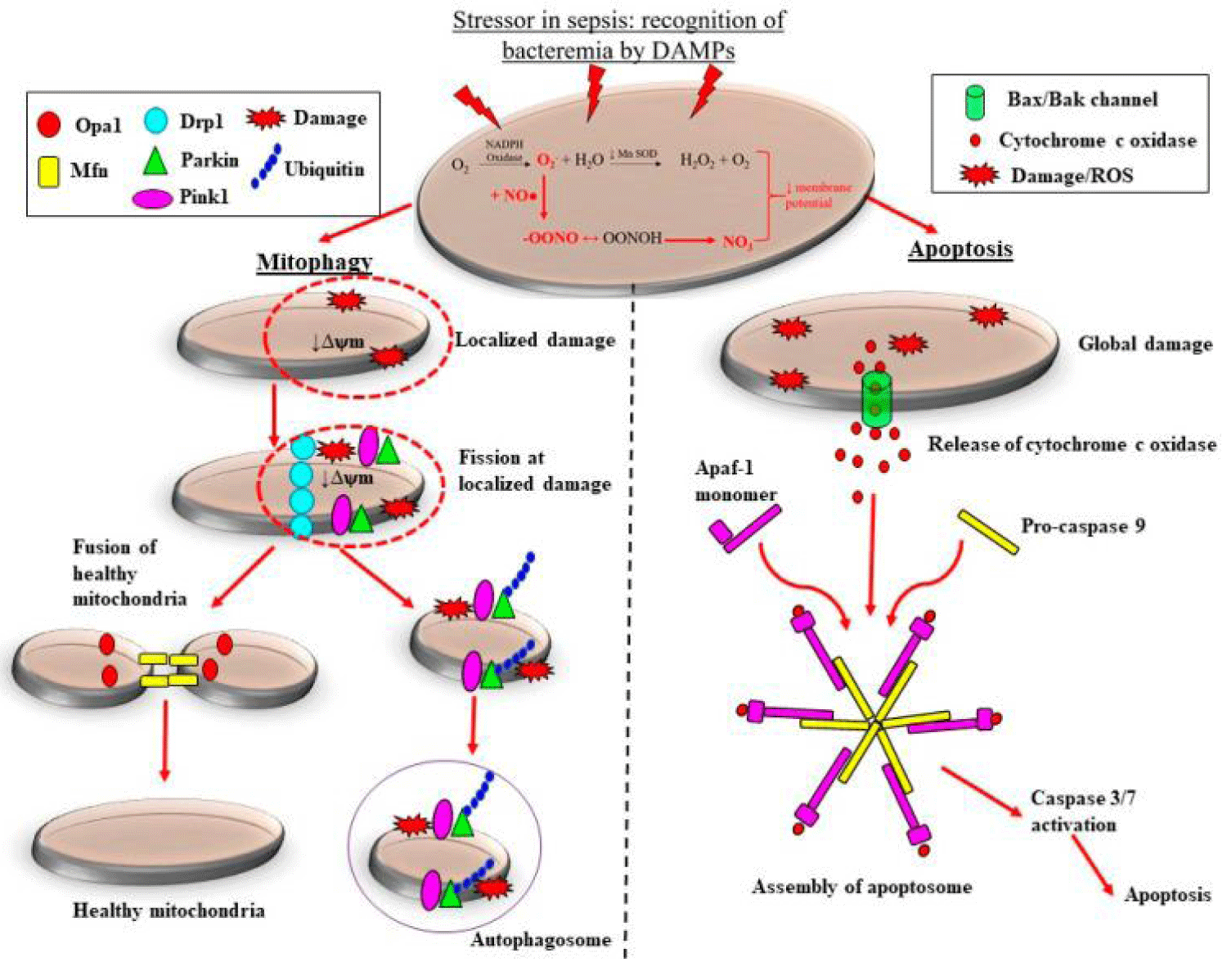
Figure 3:
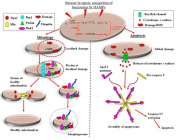
Courtesy ref no-15-Adaptive and maladaptive reactions of the mitochondria to sepsis- stimulated induced oxidative stress. Escalated generation of superoxide (O2−) by nicotinamide adenine dinucleotide phosphate (NADPH) oxidase coupled with the reduction in action of manganese superoxide dismutase (MnSOD) causes accrual of O2−. Cytosolic nitric oxide (NO) produced by inducible nitric oxide synthase (iNOS) reacts with O2−, forming the highly reactive peroxynitrite (ONOO−). The accrual of O2− and ONOO− causing continued oxidative stress along with adecrease in mitochondrial membrane potential (ψm) and so mitochondrial impairment. In the case of localized mitochondrial injury, mitochondrial quality control modes are activated which arrest mitochondrial impairment. In addition to restrict escalated mitochondrial depletion. Recruitment of mitochondrial fission proteins to sites of damage targets the injured regions of the mitochondrion for fission. Subsequently, ubiquitin, PTEN-induced kinase (PINK1) and E3 ubiquitin-protein ligase (Parkin) proteins areenrolled to the injured mitochondrion, removed by mitochondrial fission and crosstalk with phagophore, consequently generating an autophagosome. The healthy regions of the mitochondrion undergoes mitochondrial fusion, that adds to the existing mitochondrial pool and restricting escalated mitochondrial depletion. Conversely, Comprehensive injury to mitochondria in robust, sepsis causes the liberation of cytochrome c oxidase, along with in the f generation of an apoptosome when it crosstalks with apoptotic protease activating factor-1 (Apaf-1) monomers as well as pro-caspase 9. This results in downstream activation of caspase 3/7, ultimately causing the containment of sepsis-induced injury via apoptosis. Mitochondrial dynamin-like GTPase (OPA-1); dynamin related protein-1 (DRP-1); damage-associated molecular patterns (DAMPs).
Read Full Article HTML DOI: 10.29328/journal.jcn.1001084 Cite this Article Read Full Article PDF
More Images
Similar Articles
-
Acute Tubulointerstitial Nephritis due to Phenytoin: Case ReportNilzete Liberato Bresolin*,Pedro Docusse Junior,Maria Beatriz Cacese Shiozawa,Marina Ratier de Brito Moreira,Natalia Galbiatti Silveira. Acute Tubulointerstitial Nephritis due to Phenytoin: Case Report. . 2017 doi: 10.29328/journal.jcn.1001004; 1: 019-025
-
The outcome of Acute Kidney Injury in patients with severe MalariaJoão Egidio Romão Jr*,João Alberto Brandão. The outcome of Acute Kidney Injury in patients with severe Malaria. . 2017 doi: 10.29328/journal.jcn.1001007; 1: 048-054
-
Emphysematous pyelonephritis – A case series from a single centre in Southern IndiaPhanisri Alaparthi,Shobhana Nayak Rao*,Pradeep Shenoy M. Emphysematous pyelonephritis – A case series from a single centre in Southern India. . 2018 doi: 10.29328/journal.jcn.1001014; 2: 020-024
-
AngioJetTM rheolytic thrombectomy induced intravascular haemolysis leading to Acute Kidney Injury requiring DialysisHultin Sebastian*,Murali Karumathil,Nguyen Tam. AngioJetTM rheolytic thrombectomy induced intravascular haemolysis leading to Acute Kidney Injury requiring Dialysis. . 2018 doi: 10.29328/journal.jcn.1001015; 2: 025-028
-
Urine Leak Following Kidney Transplantation: An Evidence-based Management PlanShafiq A Chughtai,Ajay Sharma,Ahmed Halawa*. Urine Leak Following Kidney Transplantation: An Evidence-based Management Plan. . 2018 doi: 10.29328/journal.jcn.1001018; 2: 044-052
-
Urinary NGAL incorporation into Renal Angina Index for early detection of acute kidney injury in critically ill childrenAli Mohammed Abu Zeid,Doaa Youssef Mohammed*,Amal Saeed AbdAlazeem,Anas Saad Elsayed mohammed Seddeeq,Ashraf Mohamed Elnaany. Urinary NGAL incorporation into Renal Angina Index for early detection of acute kidney injury in critically ill children. . 2019 doi: 10.29328/journal.jcn.1001032; 3: 093-099
-
Causes of hospital admission of chronic kidney disease patient in a tertiary kidney care hospitalSalman Imtiaz*,Ruqaya Qureshi,Amna Hamid,Beena Salman,Murtaza F Drohlia,Aasim Ahmad. Causes of hospital admission of chronic kidney disease patient in a tertiary kidney care hospital. . 2019 doi: 10.29328/journal.jcn.1001033; 3: 100-106
-
Granulomatosis with polyangiitis (GPA) in a 76-year old woman presenting with pulmonary nodule and accelerating acute kidney injuryNoor Sameh Darwich*,Melissa Schnell,L. Nicholas Cossey. Granulomatosis with polyangiitis (GPA) in a 76-year old woman presenting with pulmonary nodule and accelerating acute kidney injury. . 2020 doi: 10.29328/journal.jcn.1001048; 4: 001-006
-
Biomarkers in acute kidney injuryNehomar Pajaro Galvis*,Jorge Rico-Fontalvo,Rodrigo Daza-Arnedo,Maria Ximena Cardona-Blanco,Emilio Abuabara-Franco,Victor Leal-Martínez,Jose Cabrales-Juan,José Correa-Guerrero,José Bohórquez-Rivero,José Sáenz-López,José Restom-Arrieta,Milton Rivera-Moreno,Estefany Rivera-Moreno,Maria Monterrosa-Robles,Alonso Pomares-Lara,Dayana Ayola-Rosales,Ana Alonso-Henriquez. Biomarkers in acute kidney injury. . 2020 doi: 10.29328/journal.jcn.1001056; 4: 027-035
-
Acute kidney injury in Colombian patients with COVID-19 who received kidney support therapy with genius® 90 technologyEmilio Rey-Vela,Jesús Muñoz,Rodrigo Daza-Arnedo,Rodrigo Daza-Arnedo,Katherin Portela-Buelvas,Nehomar Pájaro-Galvis*,Víctor Leal-Martínez,Emilio Abuabara-Franco,José Cabrales-Juan,Leonardo Marín,Lucas Daza,Samuel Cuadro,Emir Ortiz,María Raad-Sarabia,Cesar Ferrer,Alejandra Prada,Greisy González,Elkin Mendoza,Klearly Tinoco,Jorge Camacho,Joel Ortega,Carlos Tobón,Juan Montes,Jorge Coronado,Luis Salgado-Montiel,José Correa,Fabio Salas,Amilkar Almanza-Hurtado,Miguel Aguilar-Schorborg. Acute kidney injury in Colombian patients with COVID-19 who received kidney support therapy with genius® 90 technology. . 2020 doi: 10.29328/journal.jcn.1001059; 4: 056-060
Recently Viewed
-
Mechanism of Small Molecule Inhibitors of PhagocytosisMelika Loriamini, Melissa M Lewis-Bakker, Beth Binnington, Lakshmi P Kotra, Donald R Branch*. Mechanism of Small Molecule Inhibitors of Phagocytosis. J Hematol Clin Res. 2023: doi: 10.29328/journal.jhcr.1001022; 7: 011-014
-
Advances in Physiological Research: Consideration on Arterial Hypercapnia in Acute Cardiogenic Pulmonary Edema (ACPE)Andrea Bellone*. Advances in Physiological Research: Consideration on Arterial Hypercapnia in Acute Cardiogenic Pulmonary Edema (ACPE). Arch Case Rep. 2024: doi: 10.29328/journal.acr.1001108; 8: 116-117
-
Jaw Subluxation as a Complication of Tardive DyskinesiaDiana Paleacu Kertesz*. Jaw Subluxation as a Complication of Tardive Dyskinesia. Arch Case Rep. 2024: doi: 10.29328/journal.acr.1001112; 8: 131-133
-
Sleep Disorders and Sleep Studies Case ReportsAjay B Gadicha*,Vijay B Gadicha,Mayur S Burange,ZI Khan. Sleep Disorders and Sleep Studies Case Reports. Arch Case Rep. 2024: doi: 10.29328/journal.acr.1001114; 8: 146-151
-
Evaluation of Biostimulants Based on Recovered Protein Hydrolysates from Animal By-products as Plant Growth EnhancersH Pérez-Aguilar*, M Lacruz-Asaro, F Arán-Ais. Evaluation of Biostimulants Based on Recovered Protein Hydrolysates from Animal By-products as Plant Growth Enhancers. J Plant Sci Phytopathol. 2023: doi: 10.29328/journal.jpsp.1001104; 7: 042-047
Most Viewed
-
Evaluation of Biostimulants Based on Recovered Protein Hydrolysates from Animal By-products as Plant Growth EnhancersH Pérez-Aguilar*, M Lacruz-Asaro, F Arán-Ais. Evaluation of Biostimulants Based on Recovered Protein Hydrolysates from Animal By-products as Plant Growth Enhancers. J Plant Sci Phytopathol. 2023 doi: 10.29328/journal.jpsp.1001104; 7: 042-047
-
Sinonasal Myxoma Extending into the Orbit in a 4-Year Old: A Case PresentationJulian A Purrinos*, Ramzi Younis. Sinonasal Myxoma Extending into the Orbit in a 4-Year Old: A Case Presentation. Arch Case Rep. 2024 doi: 10.29328/journal.acr.1001099; 8: 075-077
-
Feasibility study of magnetic sensing for detecting single-neuron action potentialsDenis Tonini,Kai Wu,Renata Saha,Jian-Ping Wang*. Feasibility study of magnetic sensing for detecting single-neuron action potentials. Ann Biomed Sci Eng. 2022 doi: 10.29328/journal.abse.1001018; 6: 019-029
-
Physical activity can change the physiological and psychological circumstances during COVID-19 pandemic: A narrative reviewKhashayar Maroufi*. Physical activity can change the physiological and psychological circumstances during COVID-19 pandemic: A narrative review. J Sports Med Ther. 2021 doi: 10.29328/journal.jsmt.1001051; 6: 001-007
-
Pediatric Dysgerminoma: Unveiling a Rare Ovarian TumorFaten Limaiem*, Khalil Saffar, Ahmed Halouani. Pediatric Dysgerminoma: Unveiling a Rare Ovarian Tumor. Arch Case Rep. 2024 doi: 10.29328/journal.acr.1001087; 8: 010-013

HSPI: We're glad you're here. Please click "create a new Query" if you are a new visitor to our website and need further information from us.
If you are already a member of our network and need to keep track of any developments regarding a question you have already submitted, click "take me to my Query."